Poisons Regulations 2008
I, the Governor in and over the State of Tasmania and its Dependencies in the Commonwealth of Australia, acting with the advice of the Executive Council, make the following regulations under the Poisons Act 1971 .
8 December 2008PETER G. UNDERWOOD
Governor
By His Excellency's Command,
MICHELLE O'BYRNE
Minister for Environment, Parks, Heritage and the Arts acting for and on behalf of the Minister for Health
PART 1 - Preliminary
These regulations may be cited as the Poisons Regulations 2008 .
These regulations take effect on 1 January 2009.
(1) In these regulations, unless the contrary intention appears –Act means the Poisons Act 1971 ;ambulance officer means a person who is –(a) an officer of the ambulance service appointed or employed under section 14(2) of the Ambulance Service Act 1982 ; or(b) an honorary ambulance officer appointed under section 16 of the Ambulance Service Act 1982 ;Ambulance Service means the Tasmanian Ambulance Service established under the Ambulance Service Act 1982 ;approved first aid kit means a first aid kit kept in accordance with approval granted under regulation 92 ;approved name, in relation to a poison, restricted substance or narcotic substance means the name for that poison, restricted substance or narcotic substance determined in a manner approved by the Minister;approved recording system means a system or method approved by the Pharmacy Board of Tasmania for the keeping of records of prescriptions;authorised nurse means a nurse who holds a nurse's authority granted under regulation 31 ;authorised officer, in relation to a medical institution, means –(a) a pharmaceutical chemist employed as such in that institution or, where more than one pharmaceutical chemist is employed, the chief pharmaceutical chemist; or(b) where no pharmaceutical chemist is employed in that institution, the medical practitioner in charge of that institution; or(c) where no pharmaceutical chemist is employed in that institution and there is no medical practitioner in charge thereof, the registered nurse in charge of that institution;authorised optometrist means a person who is authorised under Division 3A of Part 3 of the Optometrists Registration Act 1994 to prescribe a restricted substance;Board means the Pharmacy Board of Tasmania;chemist means –(a) a pharmaceutical chemist; or(b) a manufacturing chemist or wholesale chemist licensed under section 16 of the Act;Commonwealth Department means the Department of State of the Commonwealth responsible for the administration of the Therapeutic Goods Act 1989 of the Commonwealth or of such Act that from time to time has effect in substitution for that Act;day book means any continuous written record kept by a medical practitioner indicating the medicines or narcotic substances supplied to patients;declared restricted substance means a restricted substance that, in accordance with an order in force under section 36 of the Act, is a substance to which that section applies;dental therapist means a person who is registered as a dental therapist under the Dental Practitioners Registration Act 2001 ;dentist means a person who is registered as a dentist under the Dental Practitioners Registration Act 2001 ;detainee means a person who is being lawfully detained in a detention centre;detention centre means a detention centre established under section 123 of the Youth Justice Act 1997 ;Director of Ambulance Services means the Director of Ambulance Services holding office under the Ambulance Service Act 1982 ;Director of Public Health means the Director of Public Health appointed under the Public Health Act 1997 ;dispensary means the room or area, within a pharmacy or other premises, that a pharmaceutical chemist uses for the dispensing or preparation of prescriptions, medicines or drugs;drug means a poison intended for human therapeutic use or animal therapeutic use;drug therapy chart means a document prepared by a medical practitioner, dentist or authorised nurse practitioner authorising the administration of a scheduled substance to a person;enrolled nurse means a person enrolled under the Nursing Act 1995 ;health professional means –(a) a dentist; and(b) a medical practitioner; and(c) a pharmaceutical chemist; and(d) a registered nurse; and(e) an authorised nurse practitioner; and(f) a veterinary surgeon;immediate wrapper means –(a) any material used as the first wrapper for a single tablet, pastille, capsule or product unit; or(b) strip packaging when used in connection with some form of primary pack;internal use, in respect of a substance, means administration orally, parenterally or by way of a body orifice but does not include the administration of topical preparations for use in the nose, eyes, ears, mouth or throat or douches for rectal, vaginal or urethral use;medical institution means an institution the sole or main object, or one of the main objects, of which is, or is held out to be, the provision of accommodation (whether with or without medical or other treatment) for –(a) persons suffering from any illness, injury, infirmity or mental disorder; or(b) pregnant women or women immediately after childbirth; or(c) persons who are substantially and permanently handicapped by illness, injury or congenital deformity, or by any other disability; or(d) persons who are aged;nurse practitioner treatment means treatment carried out by a nurse practitioner in accordance with an authorisation under section 25B of the Act;Nursing Board means the Nursing Board of Tasmania established under the Nursing Act 1995 ;optometrist means a person who is registered as an optometrist under the Optometrists Registration Act 1994 ;patient means, when used in relation to a medical practitioner, dentist, podiatrist, dental therapist, registered nurse, authorised nurse practitioner, optometrist or enrolled nurse, a person upon whom that medical practitioner, dentist, podiatrist, dental therapist, registered nurse, authorised nurse practitioner, optometrist or enrolled nurse attends in the exercise of his or her practice, profession or calling as such;pharmacy means a shop or other place, or a part of a shop or other place, in which a person practises as a pharmacist;podiatrist means a person who is registered as a podiatrist under the Podiatrists Registration Act 1995 ;primary pack means the package, other than any wrapping, bag, carton or similar article, in which any poison or restricted substance is placed for the purposes of delivery to a person after sale;Registrar of Chemical Products means the Registrar of Chemical Products appointed under the Agricultural and Veterinary Chemicals (Control of Use) Act 1995 ;selected container means –(a) a single use syringe; or(b) any other container having a capacity not exceeding 10 millilitres;specified potent substance means a substance that, in accordance with regulation 51 , is a specified potent substance for the purposes of Division 3 of Part 4 ;specified psychotropic substance means a restricted substance that, in accordance with regulation 47 , is a specified psychotropic substance for the purposes of section 38(1)(b) of the Act;trainee pharmacist means a person who is undertaking an approved registration program for the purposes of Part 2 of the Pharmacists Registration Act 2001 ;Uniform Standard means the Standard for the Uniform Scheduling of Drugs and Poisons published by the Commonwealth under the Therapeutic Goods Act 1989 of the Commonwealth, as amended from time to time.(2) In these regulations, a reference to a form followed by a number is, unless otherwise indicated, a reference to the form of that number set out in Schedule 1 .(3) A reference in these regulations to a substance includes, unless specifically exempted –(a) the substance prepared from natural sources or artificially; and(b) if the substance is a plant, other than a plant included in Schedule 8 to the Poisons List or a prohibited plant, that plant or any part of it when packed or prepared for therapeutic use; and(c) any salt, active principle or derivative of the substance, including esters and ethers, and any salt of the active principle or derivative; and(d) any alkaloid of the substance and any salt of the alkaloid; and(e) any stereo-isomer of the substance and any salt of the stereo-isomer, except if the substance is levomethorphan or levorphanol; and(f) any preparation or admixture containing any proportion of the substance or of any other substance included in paragraph (a) , (b) , (c) , (d) or (e) .(4) If a poison is present in a substance or preparation as a salt, active principle or derivative, any concentration, strength or quantity in respect of that substance or preparation is to be calculated as the concentration, strength or quantity of the poison in the form in which it is named in the Poisons List.(5) A reference in these regulations to a substance by name followed, in parentheses, by a capital letter "S" and a number is a reference to that substance when included in the correspondingly numbered Schedule to the Poisons List.
4. Preparations containing substances listed in two or more Schedules to Poisons List
(1) Subject to subregulation (2) , where a preparation contains 2 or more scheduled substances, that preparation, unless the contrary intention appears, is to be taken to be included in each of the Schedules to the Poisons List in which those substances are included.(2) Where, in relation to a preparation referred to in subregulation (1) , it is not possible to comply both with a requirement of the Act applicable to that preparation by reason of its inclusion in one Schedule to the Poisons List and with such a requirement applicable to it by reason of its inclusion in another such Schedule, the preparation, unless the contrary intention appears, is to be taken for the purposes of the Act to be included in the more restrictive of those Schedules.(3) For the purposes of subregulation (2) , the comparative restrictiveness of the Schedules to the Poisons List, in descending order, is 8, 4, 7, 1, 3, 2, 6, 5.
PART 2 - Administration
5. Manufacturing chemists and wholesale chemists
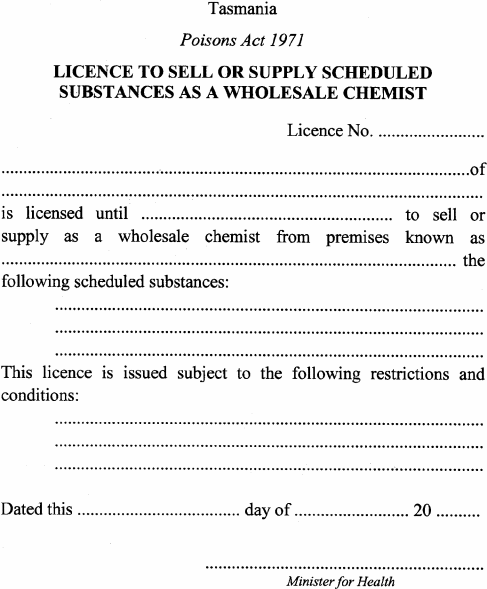
(1) An application for a licence –(a) to carry on business as a manufacturing chemist or to make, refine or prepare a narcotic substance is to be made by a qualified person and accompanied by the relevant fee specified in item 1 of Schedule 9 ; and(b) to carry on business as a wholesale chemist is to be accompanied by the relevant fee specified in item 2 of Schedule 9 .(2) A licence –(a) to carry on business referred to in subregulation (1)(a) is to be in accordance with Form 1; and(b) to carry on business referred to in subregulation (1)(b) is to be in accordance with Form 2.(3) For the purposes of –(a) section 16(2)(a) of the Act, Form 1 is prescribed; and(b) section 16(3)(a) of the Act, Form 2 is prescribed.(4) In subregulation (1)(a) –qualified person means –(a) a medical practitioner, pharmaceutical chemist, dentist or veterinary surgeon; or(b) a person who holds a degree or diploma approved by the Minister; or(c) a person approved by the Secretary; or(d) a person who is, or is eligible to be –(i) a Fellow or Associate of the Royal Australian Chemical Institute; or(ii) a Fellow, Associate or Licentiate of the Royal Institute of Chemistry; or(e) where the carrying out of a process involving a prohibited plant is only preparatory to the use of that plant for the manufacture of a narcotic substance, a person approved by the Minister.
6. Granting of permits by Minister
(1) The Minister may, on the application of a person and on payment of the fee (if any) specified in Schedule 9 , grant a permit under this regulation.(2) A permit under subregulation (1) authorises the person to whom it is issued to purchase from a licensed manufacturing chemist or a licensed wholesale chemist any of the substances specified in Schedule 1 , 2 , 3 or 4 to the Poisons List in such quantities and on such conditions, limitations and restrictions as the Minister may determine and as may be specified in the permit for use by the person for industrial, educational, advisory or research purposes.(3) A permit issued under subregulation (1) remains in force until it is cancelled, suspended or revoked by the Minister.
PART 3 - Narcotic Substances and Prohibited Plants
Division 1 - Licences in respect of narcotic substances and prohibited plants
7. Licences to manufacture, &c., narcotic substances for scientific purposes
(1) The Minister may, on the application of a person –(a) who is in charge of a laboratory for the purpose of research or instruction; or(b) who is an analyst appointed under section 19 of the Act –grant a licence authorising that person –(c) to manufacture, use or possess any narcotic substance specified in the licence for such purpose as is specified in the licence; or(d) to purchase that narcotic substance by an order written in ink from a pharmaceutical chemist, a licensed manufacturing chemist, a licensed wholesale chemist or such other person as may be specified in the licence.(2) The holder of a licence granted under subregulation (1) is to keep a record in the form and manner approved by the Secretary showing –(a) the amount of narcotic substance acquired for use under the licence; and(b) the date on which, and the source from which, the narcotic substance was acquired; and(c) the purpose for which, the amount of, and the date on which, the narcotic substance was used.
The annual fees specified in Schedule 9 are prescribed as the annual fees that are payable in respect of the several matters to which they respectively relate.
Division 2 - Possession of narcotic substances
9. Possession of narcotic substances for purposes of profession, &c.
In addition to the persons authorised by section 48 of the Act to possess narcotic substances, a person who is –may possess and use any narcotic substances for the purposes of his or her profession or employment.(a) an authorised officer of a medical institution; or(b) a registered nurse in charge of a ward in a medical institution; or(c) an authorised nurse; or(d) an ambulance officer –
Division 3 - Supply of narcotic substances
10. Inquiries, &c., before supplying narcotic substance to patient
(1) A medical practitioner, dentist, authorised nurse practitioner or authorised nurse must not supply, or write or issue a prescription for the supply of, a narcotic substance to a person unless the medical practitioner, dentist, authorised nurse practitioner or authorised nurse has taken such steps as are reasonably open to him or her to ascertain –(a) the nature and amount of any narcotic substances supplied to that person within the previous 2 months; and(b) the circumstances in which those narcotic substances were so supplied.Penalty: Fine not exceeding 10 penalty units.(2) A person who obtains from, or as a result of a prescription written or issued by, a medical practitioner, dentist or authorised nurse practitioner a narcotic substance by failing, before or at the material time, to notify the medical practitioner, dentist or authorised nurse practitioner the name and the place of practice of a medical practitioner, dentist or authorised nurse practitioner by whom within a period of 2 months before the material time a narcotic substance was supplied to the person, or a prescription for a narcotic substance was written or issued in respect of the person, is guilty of an offence.Penalty: Fine not exceeding 10 penalty units.(3) Subregulations (1) and (2) do not apply to the supply of a narcotic substance to a medical practitioner, dentist, authorised nurse practitioner, licensed wholesale chemist, licensed manufacturing chemist or pharmaceutical chemist, otherwise than for the purpose of its administration to the person to whom it is supplied.(4) In this regulation –material time, when used in relation to the obtaining of –(a) a narcotic substance from a medical practitioner, veterinary surgeon, dentist, authorised nurse practitioner or authorised nurse, means the time at which that substance was obtained; or(b) a narcotic substance as a result of a prescription written or issued by a medical practitioner, dentist or authorised nurse practitioner, means the time at which that prescription was written or issued; or(c) a prescription for a narcotic substance from a medical practitioner, veterinary surgeon, dentist or authorised nurse practitioner, means the time at which the prescription was obtained.
11. Supply of narcotic substances
(1) A person must not supply a narcotic substance to a person who is not authorised by the Act or these regulations to have possession of that narcotic substance.Penalty: Fine not exceeding 10 penalty units.(2) Except as otherwise provided in these regulations, a pharmaceutical chemist must not supply a narcotic substance to a person who is not a medical practitioner, veterinary surgeon, dentist, authorised nurse practitioner, licensed manufacturing chemist, licensed wholesale chemist or pharmaceutical chemist, except on a prescription issued by a medical practitioner, veterinary surgeon, dentist or authorised nurse practitioner.Penalty: Fine not exceeding 10 penalty units.(3) Except as otherwise provided in these regulations, a licensed manufacturing chemist or licensed wholesale chemist must not supply, or cause or permit to be supplied, a narcotic substance to a person who is not a medical practitioner, veterinary surgeon, dentist, authorised nurse practitioner or pharmaceutical chemist.Penalty: Fine not exceeding 10 penalty units.(4) A medical practitioner, dentist, authorised nurse practitioner or authorised nurse must not supply a narcotic substance to a person except for the purpose of its administration to a patient.Penalty: Fine not exceeding 10 penalty units.(5) A veterinary surgeon must not supply a narcotic substance to a person except for the purpose of its administration to an animal.Penalty: Fine not exceeding 10 penalty units.(6) Nothing in subregulation (4) or (5) prohibits a medical practitioner, veterinary surgeon, dentist, authorised nurse practitioner or authorised nurse from supplying a narcotic substance to a medical practitioner, veterinary surgeon, dentist, authorised nurse practitioner, licensed wholesale chemist, licensed manufacturing chemist or pharmaceutical chemist.(7) Where a person supplies a narcotic substance on a written order, the person must –(a) write clearly in ink on the order the word "Cancelled"; and(b) retain that order in a file kept for the purpose for a period of 2 years from the date on which the narcotic substance was supplied.Penalty: Fine not exceeding 10 penalty units.(8) This regulation does not apply to a narcotic substance that is kept or used in a prescribed institution, within the meaning of section 48 of the Act, for the purposes of that institution or to a narcotic substance which is kept or used under a licence granted under regulation 7 .(9) Nothing in this regulation prohibits the administration to a person, in the case of an emergency, of a narcotic substance kept in any aircraft, ambulance or vessel where –(a) the services of a medical practitioner are not readily available; or(b) it is not practicable to obtain the narcotic substance from any other source.(10) Nothing in this regulation prohibits a pharmaceutical chemist, licensed wholesale chemist or licensed manufacturing chemist from supplying any narcotic substance to –(a) an authorised officer in a medical institution for the use of the narcotic substance for the purpose of that institution; or(b) a person holding a licence under regulation 7 ; or(c) an authorised nurse; or(d) a person holding the Secretary's written authority for use in respect of an ambulance service; or(e) a person controlling an approved first aid kit; or(f) the master of a vessel for the purpose of providing medical treatment to passengers or crew of that vessel.
12. Conveyance of narcotic substances
A person may possess a narcotic substance for the purpose of conveying it to any person or place if –(a) the narcotic substance is contained in a sealed package or container; and(b) the person is acting –(i) in the course of the person's business or employment to carry, convey or deliver articles or containers of a similar nature; or(ii) under the directions of a person authorised by the Act to have possession of that narcotic substance.
Division 4 - Records and returns as to narcotic substances
13. Narcotic substances register to be kept by certain persons
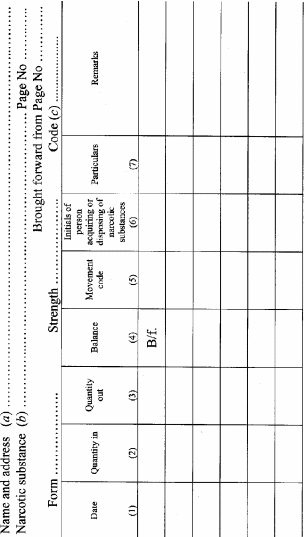
(1) The following people must keep a narcotic substances register in accordance with this regulation:(a) a medical practitioner;(b) a veterinary surgeon;(c) a dentist;(d) a pharmaceutical chemist;(e) a licensed manufacturing chemist;(f) a licensed wholesale chemist;(g) an authorised officer;(h) an authorised nurse practitioner;(i) an authorised nurse.Penalty: Fine not exceeding 10 penalty units.(2) A separate narcotic substances register is to be kept in respect of each type or kind of narcotic substance or any preparation of a narcotic substance.(3) A pharmaceutical chemist must keep a separate narcotic substances register in respect of each of the premises in which he or she carries on business as a pharmaceutical chemist.(4) Where a medical practitioner, veterinary surgeon, dentist or authorised nurse practitioner carries on practice at 2 or more premises, he or she must keep a separate narcotic substances register in respect of each of those premises.(5) A narcotic substances register must be in the form set out in Part 1 of Schedule 2 and must be kept in accordance with the rules contained in Part 2 of that Schedule.(6) Notwithstanding subregulation (5) , a person referred to in subregulation (1) may use a narcotic substances register other than the register referred to in subregulation (5) if the other narcotic substances register –(a) is in a form approved by the Secretary; and(b) is kept in such manner as the Secretary may direct.(7) Each entry, marking, number or note made in a narcotic substances register is to be made in ink.(8) No alteration, obliteration or cancellation may be made in any narcotic substances register, but any mistake in any entry in the register may be corrected by a marginal note or footnote and the correction initialled and dated.(9) A person must not make any entry in a narcotic substances register if the person knows the entry to be false or misleading.Penalty: Fine not exceeding 10 penalty units.(10) Each entry in a narcotic substances register is to be made as soon as practicable after the occurrence of the event to which it relates and, in any event, not later than 48 hours after the occurrence of that event.(11) Notwithstanding anything in this regulation, where a medical practitioner, other than a dispensing medical practitioner, who supplies a narcotic substance to a patient, keeps a day book, it is sufficient compliance with the provisions of this regulation if an entry containing all particulars in respect of the supply of that narcotic substance to the patient, which apart from this subregulation would be required to be made in the narcotic substances register, were made in the day book.(12) Where the Secretary requests by a notice in writing any person required by these regulations to keep a narcotic substances register to furnish to the Secretary any information relating to the keeping of the register, that person must as soon as practicable comply with the request.(13) Nothing in these regulations requires a person who is required to keep a narcotic substances register to make any entry in respect of a narcotic substance supplied to the person by a medical practitioner, veterinary surgeon, dentist, authorised nurse practitioner or authorised nurse if the narcotic substance is for the person's personal use.
(1) A person licensed or authorised under these regulations to manufacture, sell or supply any narcotic substance must keep every record, prescription, invoice and other document relating to any transaction involving any narcotic substance for not less than 2 years from the latest date on which such record, prescription, invoice, order or document was made or acted upon.Penalty: Fine not exceeding 10 penalty units.(2) On demand by an inspector –must furnish particulars of the quantity of any narcotic substance obtained and the amount disposed of or on hand.(a) the holder of any licence to manufacture, sell or supply any narcotic substance; or(b) any person authorised by regulation 9 to possess and use a narcotic substance –Penalty: Fine not exceeding 10 penalty units.
Division 5 - Prescriptions for narcotic substances
15. Prescribing and supply of narcotic substances
(1) A person, other than a medical practitioner, veterinary surgeon, dentist or authorised nurse practitioner, must not write or issue a prescription for a narcotic substance.Penalty: Fine not exceeding 10 penalty units.(2) A medical practitioner, veterinary surgeon or dentist, subject to this regulation, is authorised to write or issue a prescription for a narcotic substance.(3) An authorised nurse practitioner may write or issue a prescription for a narcotic substance in such circumstances, subject to such conditions and in relation to such substances or classes of substances as may be specified in an authorisation issued by the Secretary.(4) A person must not write or issue a prescription for the purpose of procuring a narcotic substance for administration to himself or herself.Penalty: Fine not exceeding 10 penalty units.(5) A person must not write or issue a prescription for a narcotic substance unless the prescription includes (otherwise than in handwriting) the name of the person writing or issuing the prescription and the address of the person's place of residence or of the place at which the person carries on practice.Penalty: Fine not exceeding 10 penalty units.(6) Subregulation (5) does not apply in the case of an emergency when the means of complying with that subregulation are not readily available to the person writing or issuing the prescription.(7) Subject to regulation 16 , where a medical practitioner, veterinary surgeon, dentist or authorised nurse practitioner writes or issues a prescription for a narcotic substance, he or she must comply with the following conditions:(a) he or she is to legibly write the prescription in ink and, in his or her own handwriting, is to include in the prescription –and sign the prescription with his or her usual signature;(i) the date on which it is written; and(ii) the name, including initials and address, of the patient or, in the case of an animal, the name and address of the owner; and(iii) the name of the narcotic substance and quantity to be dispensed; and(iv) subject to subregulation (9) , the number of times that the dispensing of the prescription may be repeated and the interval between each dispensing of the prescription; and(v) adequate directions for use –(b) he or she is not to include in that prescription a preparation other than the preparation which is or includes the narcotic substance;(c) if a medical practitioner, he or she is not to write or issue a prescription for the supply of a narcotic substance for any purpose other than in the course of medical treatment;(d) if a veterinary surgeon, he or she is not to write or issue a prescription for the supply of a narcotic substance for any purpose other than in the course of animal treatment and is to include in a prescription which he or she issues the words "For animal treatment only";(e) if a dentist, he or she is not to write or issue a prescription for the supply of a narcotic substance for any purpose other than in the course of dental treatment;(f) if an authorised nurse practitioner, he or she is not to write or issue a prescription for the supply of a narcotic substance for any purpose other than in the course of nurse practitioner treatment;(g) where the prescription contains an unusual dose, the medical practitioner, veterinary surgeon, dentist or authorised nurse practitioner by whom it is written or issued is to underline, or by some other means emphasise, that part of the prescription and, except for a prescription issued in accordance with an approval under regulation 16 , sign or initial that part in the margin.Penalty: Fine not exceeding 10 penalty units.(8) Notwithstanding subregulation (6)(a)(ii) , a medical practitioner, veterinary surgeon, dentist or authorised nurse practitioner may use a printed label in a prescription to identify the patient or owner of the animal, if that label is initialled by the medical practitioner, veterinary surgeon, dentist or authorised nurse practitioner.(9) A dentist must not write or issue a prescription for a narcotic substance that may be dispensed on more than one occasion.Penalty: Fine not exceeding 10 penalty units.(10) A person must not write or issue a prescription for a narcotic substance which, or any part of which, is in a code or cipher.Penalty: Fine not exceeding 10 penalty units.(11) A veterinary surgeon must not prescribe or supply any of the narcotic substances specified in regulation 19(1) otherwise than in accordance with an authorisation under subregulation (12) .Penalty: Fine not exceeding 10 penalty units.(12) The Secretary may, either generally or in a particular case, authorise a veterinary surgeon to prescribe or supply methylphenidate for veterinary purposes.(13) An authorisation under subregulation (12) –(a) is to be in writing signed by the Secretary; and(b) in the case of a general authorisation, is to require that, within 24 hours of each occasion on which the veterinary surgeon prescribes or supplies methylphenidate, the veterinary surgeon send to the Secretary notice in writing specifying –(i) the name and address of the owner of the animal for the treatment of which the prescription was given or the methylphenidate was supplied; and(ii) the amount of methylphenidate prescribed or supplied; and(iii) the date of the prescription or supply; and(c) may specify such other conditions to which it is subject as the Secretary thinks fit; and(d) may, at any time, be revoked or amended by the Secretary by notice in writing given to the veterinary surgeon.(14) A dentist must not prescribe for, or supply to, a person any of the narcotic substances specified in regulation 19(1) .Penalty: Fine not exceeding 10 penalty units.
16. Electronic prescriptions for narcotic substances
(1) Where a medical practitioner, veterinary surgeon, dentist or authorised nurse practitioner is authorised to issue a prescription for a narcotic substance, that prescription may be issued electronically if the Secretary so approves.(2) For the purpose of subregulation (1) , issuing a prescription electronically includes issuing the prescription by transmitting data electronically without producing a printed copy of that data.(3) An approval under subregulation (1) –(a) is to be in writing signed by the Secretary; and(b) may be of general or specific application; and(c) may be made subject to such conditions as the Secretary thinks fit; and(d) may be amended or revoked by the Secretary by notice in writing.(4) The conditions specified in regulation 15(7) , with the exception of the condition that a prescription be handwritten in ink, apply to the issuing of a prescription electronically in accordance with an approval under subregulation (1) .(5) For the purposes of regulation 15(7)(a) as it applies to the issuing of a prescription in accordance with an approval under subregulation (1) , a signature includes an electronic signature.
17. Record of prescribing and supply of narcotic substances
(1) As soon as practicable after a medical practitioner, dentist, authorised nurse practitioner or veterinary surgeon issues a prescription for, or supplies, a narcotic substance, he or she must make a record, in a form approved by the Secretary, setting out –(a) the name and address of the person for the treatment of whom, or of the owner of the animal for the treatment of which, the narcotic substance was prescribed or supplied; and(b) the date on which the prescription was issued or the narcotic substance was supplied, as the case requires; and(c) particulars of the narcotic substance sufficient to identify it and to indicate in what quantity and strength it was prescribed or supplied; and(d) particulars of the directions set out in the prescription, or provided with the narcotic substance, for the use of the narcotic substance; and(e) where the record relates to the issue of a prescription, particulars of any provision made in the prescription in respect of the number of occasions on which, and the minimum intervals at which, the dispensing of the prescription was authorised to be repeated.Penalty: Fine not exceeding 10 penalty units.(2) A medical practitioner, dentist, authorised nurse practitioner or veterinary surgeon must retain a record made under subregulation (1) for not less than 2 years.Penalty: Fine not exceeding 10 penalty units.
18. Emergency prescribing and dispensing of narcotic substances
(1) Notwithstanding regulation 11(2) , a pharmaceutical chemist may supply a narcotic substance on the instruction of a medical practitioner, veterinary surgeon, dentist or authorised nurse practitioner if, because of the urgent circumstances in which the substance is required, it is impracticable, before the substance is required to be supplied, to –(a) issue a prescription for the substance; and(b) cause the prescription to be delivered to the chemist.(2) A medical practitioner, veterinary surgeon, dentist or authorised nurse practitioner who issues an instruction under subregulation (1) to a pharmaceutical chemist must send to that chemist, within 24 hours of issuing the instruction, a prescription that –(a) complies with regulation 15(7) ; and(b) clearly states that it is in confirmation of the instruction to that chemist to supply the narcotic substance without a prescription.
19. Prescription for certain narcotic substances to be issued only on authority of Secretary
(1) A medical practitioner must not, without the authority of the Secretary, issue a prescription for, or supply to a patient, the following narcotic substances, namely:(a) amphetamine;(b) dexamphetamine;(c) dextromoramide;(d) fentanyl in patches for transdermal delivery, except in the case of a patient with cancer;(e) fentanyl lozenges, except in the case of a patient with cancer;(f) methylamphetamine;(g) methylphenidate;(h) phenmetrazine.Penalty: Fine not exceeding 10 penalty units.(2) An application for an authority to issue a prescription for a narcotic substance referred to in subregulation (1) , or to supply to a patient a narcotic substance referred to in that subregulation –(a) is to be in a form determined by the Secretary; and(b) is to be signed by the medical practitioner by whom it is made; and(c) is to –(i) specify the patient in respect of whom it is made; and(ii) state whether, in the opinion of the medical practitioner, the patient is suffering from drug dependency; and(d) where in the opinion of the medical practitioner the patient in respect of whom the application is made is suffering from drug dependency, is to be accompanied by a notification under section 18 of the Alcohol and Drug Dependency Act 1968 ; and(e) is to be enclosed in a sealed envelope marked "Confidential" and lodged with, or forwarded by certified mail to, the Secretary.(3) An authority under this regulation is to be in writing and signed by the Secretary unless, in the case of emergency, it is given orally.(4) An authority that is given orally is to be confirmed in writing by the Secretary as soon as practicable after it is given.(5) An authority under this regulation is to specify the type and quantity of the narcotic substance that may –in accordance with the terms of the authority by the medical practitioner to whom the authority relates.(a) be included in a prescription issued; or(b) be supplied to a patient –(6) An authority under this regulation authorises the medical practitioner to whom the authority is given or some other medical practitioner authorised by him or her in writing –in accordance with the terms of the authority.(a) to issue a prescription for a narcotic substance referred to in subregulation (1) for the use of the patient to whom the prescription relates; or(b) to supply to a patient a narcotic substance referred to in that subregulation for the use of that patient –(7) In this regulation –drug dependency has the meaning assigned to that expression by section 4 of the Alcohol and Drug Dependency Act 1968 .
20. Supply of certain narcotic substances without prior authority of Secretary
(1) Notwithstanding the provisions of regulation 19 , the Secretary may issue a general authorisation to a medical practitioner to supply to a patient a narcotic substance referred to in regulation 19(1) without seeking the prior approval of the Secretary.(2) A general authorisation under this regulation is to –(a) be in writing and be signed by the Secretary; and(b) state the purposes for which the narcotic substance may be used; and(c) require that, within 7 days of a narcotic substance referred to in regulation 19(1) being supplied to a patient, the medical practitioner advise the Secretary in writing of –(i) the name and address of the patient supplied; and(ii) the name and amount of the narcotic substance supplied; and(iii) the date of the supply.(3) The Secretary may at any time in writing revoke or amend a general authorisation issued under this regulation.
21. Registered nurse may possess and administer narcotic substance
A registered nurse may possess and administer to a person a narcotic substance without instructions from a doctor if –(a) the nurse is attending an emergency in a remote area and the person requires urgent treatment with medication; and(b) it is not practicable to obtain instructions from a doctor; and(c) the nurse has –(i) undergone an educational program approved by the Nursing Board of Tasmania; and(ii) has been authorised by that Board; and(d) the nurse follows appropriate procedures approved by the Secretary.
22. Minister’s authorisation for possession and supply of narcotic substance
The Minister may make an authorisation under section 25A of the Act in respect of a narcotic substance, or a class of narcotic substances, in the following circumstances:(a) where the registered nurse in respect of whom the authorisation is made is employed in a palliative care service approved by the Secretary;(b) where the registered nurse is employed in a community health centre approved by the Secretary at which it is impractical for a medical practitioner to attend and the nurse is acting in accordance with the instructions of a medical practitioner;(c) where the registered nurse is employed in a medical institution approved by the Secretary at which it is impractical for medical practitioners to attend after hours and the nurse is acting in accordance with the instructions of a medical practitioner.
Division 6 - Dispensing of narcotic substances
23. Dispensing of narcotic substances (S8)
(1) A person must not dispense a narcotic substance except under regulation 18 or in accordance with a prescription written and issued in accordance with regulation 15 .Penalty: Fine not exceeding 10 penalty units.(2) If a prescription for a narcotic substance directs that the substance be dispensed more than once –(a) the person responsible for dispensing the substance is to retain the prescription at the place at which the substance is dispensed; and(b) any subsequent dispensing of the substance is to be from the same place as the original dispensing.(3) Subregulation (2) does not apply if a person approved by the Secretary authorises the prescription to be transferred to another pharmacy.(4) An authorisation may be –(a) oral or written; and(b) subject to conditions.(5) Where a prescription for a narcotic substance directs that substance to be dispensed more than once, but does not specify the minimum intervals at which that prescription may be dispensed –(a) a person may dispense that prescription if –(i) the prescription conforms in all other respects with regulation 15(7) ; and(ii) that person ascertains, in accordance with subregulation (6) , the earliest date on which the dispensing of the prescription may be repeated; and(iii) on dispensing the prescription that person clearly and indelibly marks on it, over his or her signature and the date, a note specifying both the date so ascertained and how it was ascertained; and(b) a person who is unable to ascertain, in accordance with subregulation (6) , the earliest date on which the dispensing of the prescription may be repeated may dispense that prescription not more than once if –(i) the prescription conforms in all other respects with regulation 15(6) ; and(ii) on dispensing the prescription that person clearly and indelibly marks the word "cancelled" on the prescription.(6) The date referred to in subregulation (5)(a)(ii) is to be ascertained by reference to either, or both, of the following:(a) any specific directions incorporating a dosage rate that are included in the prescription for the use of that narcotic substance by the patient;(b) information obtained directly from the prescriber as to the minimum intervals at which that narcotic substance should be dispensed to the patient.(7) If the date referred to in subregulation (5)(a)(ii) is ascertained by reference only to the information referred to in paragraph (a) of subregulation (6) , that date is to be a date not earlier than 4 days before the day on which the quantity of the narcotic substance being dispensed would be exhausted if it is used strictly in accordance with the directions referred to in that paragraph.(8) A person must not dispense a prescription for a narcotic substance unless –(a) the person is a medical practitioner or registered pharmaceutical chemist; or(b) the person dispenses the prescription under the supervision of a medical practitioner or registered pharmaceutical chemist.Penalty: Fine not exceeding 10 penalty units.(9) A person must not cause or permit a person to dispense a prescription contrary to subregulation (1) or (8) .Penalty: Fine not exceeding 10 penalty units.(10) In a proceeding for an offence committed or alleged to have been committed under subregulation (1) , it is a defence to show that the defendant believed, on reasonable grounds, that the prescription was written and issued in accordance with regulation 15 .(11) Nothing in subregulation (8) prevents the dispensing of a prescription for a narcotic substance by or under the supervision of a veterinary surgeon, if the narcotic substance is intended for administration to an animal.(12) A person must not dispense, or cause or allow to be dispensed, a prescription for a narcotic substance if the person knows, or has reason to believe, or if it appears from the markings made on the prescription, that the prescription has already been dispensed, unless the prescription has been issued by a medical practitioner or authorised nurse practitioner who has indicated on the prescription in accordance with regulation 15 that the prescription may be dispensed more times than once.Penalty: Fine not exceeding 10 penalty units.(13) Without prejudice to the foregoing provisions of this regulation, a person must not dispense, or cause or allow to be dispensed, a prescription for a narcotic substance on which it is indicated that that prescription may be dispensed more times than once, if the person knows, or has reason to believe, or if it appears from the markings on the prescription, that the prescription has already been dispensed the number of times indicated on the prescription.Penalty: Fine not exceeding 10 penalty units.(14) The person by whom a narcotic substance is dispensed on a prescription and, if in so dispensing that narcotic substance that person is acting in the employment of some other person, that other person must ensure that, before the narcotic substance so dispensed is supplied to any person, there is clearly marked in ink on the prescription –(a) the date on which it is dispensed; and(b) the signature of the medical practitioner, veterinary surgeon, dental practitioner or pharmaceutical chemist by whom it is dispensed; and(c) the address of that person's residence or place of business, or the name and address of the residence or place of business of the person in whose employment that person was acting when that person dispensed the prescription; and(d) the word "cancelled", unless –(i) the medical practitioner, authorised nurse practitioner or veterinary surgeon by whom the prescription was issued has indicated on the prescription in accordance with regulation 15(7)(a)(iv) that the prescription may be dispensed on more than one occasion; and(ii) it appears from the prescription and the markings made on it that the prescription may be dispensed on a further occasion.Penalty: Fine not exceeding 10 penalty units.(15) A person must not dispense a narcotic substance under a prescription which –(a) is illegible or defaced; or(b) is marked "cancelled"; or(c) appears to have been altered; or(d) appears to be fraudulent in any respect or forged.Penalty: Fine not exceeding 10 penalty units.(16) A person to whom a prescription referred to in subregulation (15) is presented must –(a) retain the prescription notwithstanding that the prescription is not dispensed; and(b) as soon as practicable, inform the Secretary or a police officer of the relevant circumstances and the person's reasons for not dispensing the prescription.Penalty: Fine not exceeding 10 penalty units.(17) A person must not dispense a narcotic substance under a prescription which is presented more than 6 months after the date on which the prescription was issued.Penalty: Fine not exceeding 10 penalty units.(18) A medical practitioner, veterinary surgeon, dentist or registered pharmaceutical chemist who, in the course of the practice or business carried on by him or her as such, dispenses, or causes or permits to be dispensed, a prescription that is required by this regulation to be marked with the word "cancelled", must keep that prescription in a file kept for that purpose for 2 years from the date on which it is dispensed.Penalty: Fine not exceeding 10 penalty units.(19) Where a prescription is dispensed by or under the supervision of a medical practitioner, veterinary surgeon or dentist, he or she must mark, or cause to be marked, in ink on the package or container in which the narcotic substance is supplied –in respect of the supply of that narcotic substance on that prescription.(a) a reference to the entry in the narcotic substances register, if an entry is made in the narcotic substances register; or(b) a reference to the entry in the day book, if an entry is made in the day book –Penalty: Fine not exceeding 10 penalty units.(20) A registered pharmaceutical chemist who, in the course of the business of a pharmaceutical chemist carried on by the pharmaceutical chemist, dispenses, or causes or permits to be dispensed, a prescription for a narcotic substance must mark, or cause to be marked, in ink on the package or container in which the narcotic substance is supplied a reference to the entry in the prescription book of the dispensing of that prescription.Penalty: Fine not exceeding 10 penalty units.(21) Where, under the requirements of any law of the Commonwealth or of any Department of the Commonwealth, a prescription for a narcotic substance is required to be issued in duplicate and one of the copies is delivered or sent to any authority or person in accordance with those requirements, this regulation applies only to the other of those copies, and that copy is taken to be a prescription for a narcotic substance for the purposes of this regulation.(22) A person must not dispense, or cause or permit to be dispensed, a repeat of a prescription for a narcotic substance at an interval of time less than that indicated on the prescription.Penalty: Fine not exceeding 10 penalty units.(23) For the purposes of subregulation (19) –narcotic substances register means the narcotic substances register referred to in regulation 13 .(24) For the purposes of this regulation, 2 or more prescriptions issued to the same person in respect of the same narcotic substance are to be treated as a single prescription that directs that the substance may be dispensed more than once.
24. Restriction on dispensing narcotic substances
(1) A person who is unable to verify that a prescription for a narcotic substance is authentic must dispense no more of that substance than is sufficient for 2 days' treatment if it is used in accordance with the instructions on the prescription.Penalty: Fine not exceeding 10 penalty units.(2) For the purposes of subregulation (1) , a person is to be taken to have authenticated a prescription if that person –(a) is familiar with the handwriting of the purported prescriber and is satisfied that the prescription is in that handwriting; or(b) verifies with the purported prescriber that he or she wrote the prescription.
Division 7 - Storage of narcotic substances
25. Storage of narcotic substances
(1) A person who is authorised by the Act or these regulations to possess narcotic substances for the purposes of the person's profession or employment –(a) must keep them stored apart from other goods in an enclosure that is constructed and secured in a manner approved by the Secretary; and(b) when the narcotic substance is not being used, must keep the enclosure securely locked and retain the key either on his or her person or in a place not readily accessible to other persons.(2) For the purposes of subregulation (1) –goods does not include –(a) money or negotiable instruments; or(b) declared restricted substances stored in accordance with regulation 58 .(3) Subregulation (1) does not apply to or in respect of an ambulance officer.(4) Notwithstanding subregulation (1) , a medical practitioner, veterinary surgeon, dentist or authorised nurse practitioner may, for emergency purposes, keep narcotic substances in a bag in a vehicle or room which is kept securely locked when the vehicle or room is not occupied by that medical practitioner, veterinary surgeon, dentist or authorised nurse practitioner.
Division 8 - Storage and supply of narcotic substances in medical institutions
26. Authorised officer to store, supply, &c., narcotic substances in medical institution
The authorised officer in a medical institution must –(a) receive all narcotic substances supplied to that institution for the purposes of that institution; and(b) store those narcotic substances in accordance with regulation 25 ; and(c) supply those narcotic substances in accordance with these regulations; and(d) keep a narcotic substances register in respect of those narcotic substances in accordance with regulation 13 .Penalty: Fine not exceeding 10 penalty units.
27. Supply of narcotic substances in medical institution
(1) If the authorised officer in a medical institution is a pharmaceutical chemist, he or she must not supply a narcotic substance otherwise than for its administration –(a) on a prescription written in accordance with the provisions of regulation 15 or the written request of –(i) the registered nurse in charge of the ward in which the narcotic substance is to be used or stored; or(ii) an authorised nurse practitioner; or(iii) a medical practitioner; and(b) to a patient in that ward.Penalty: Fine not exceeding 10 penalty units.(2) If the authorised officer in a medical institution is the medical officer in charge or registered nurse in charge of that institution, he or she must not supply a narcotic substance otherwise than for its administration –(a) on the written request of –(i) the registered nurse in charge of the ward in which the narcotic substance is to be used or stored; or(ii) a dentist; or(iii) an authorised nurse practitioner; or(iv) a medical practitioner; and(b) to a patient in that ward.Penalty: Fine not exceeding 10 penalty units.
28. Receipt for supply of narcotic substance in medical institution
Where an authorised officer supplies a narcotic substance to any person in accordance with a written request under regulation 27 , the authorised officer must obtain from that person a receipt for the substance at the time of supply.Penalty: Fine not exceeding 10 penalty units.
29. Storage and control of narcotic substances in wards of medical institutions
(1) The registered nurse in charge of a ward of a medical institution must –(a) keep the narcotic substances supplied to that ward stored apart from all other goods, other than declared restricted substances, in a separate cupboard or receptacle that is securely fixed to the premises; and(b) keep that cupboard or receptacle securely locked at all times when the substances in it are not being used.Penalty: Fine not exceeding 10 penalty units.(2) The registered nurse in charge of a ward must keep, in accordance with this regulation, a narcotic substances register to be known as a "ward narcotic substances register" with pages numbered consecutively for each type or kind of preparation of narcotic substance supplied to the ward.Penalty: Fine not exceeding 10 penalty units.(3) A ward narcotic substances register is to be kept in accordance with the form contained in Part 1 of Schedule 3 and in accordance with the rules contained in Part 2 of that Schedule.(4) Notwithstanding subregulation (3) , the person in charge of a medical institution may authorise the registered nurse in charge of a ward in the medical institution to use a ward narcotic substances register other than the register referred to in that subregulation if the other ward narcotic substances register –(a) is in a form approved by the Secretary; and(b) is kept in such manner as the Secretary may direct.(5) Every entry, marking, number or note made in or on a ward narcotic substances register is to be made in ink as soon as practicable after the occurrence of the event to which it relates but not later than 24 hours from the occurrence of that event.
30. Administration of narcotic substances (S8) in medical institutions
(1) Subject to subregulation (2) and regulation 94 , a medical practitioner, dentist or authorised nurse practitioner must not give instructions for a narcotic substance to be administered to a patient in a medical institution without completing and signing, in his or her own handwriting or in a manner approved by the Secretary, an authorisation to do so.Penalty: Fine not exceeding 10 penalty units.(2) Nothing in subregulation (1) prohibits a medical practitioner, dentist or authorised nurse practitioner from –(a) giving verbal instructions for a narcotic substance to be administered to a patient in a medical institution in an emergency if the medical practitioner, dentist or authorised nurse practitioner subsequently complies with subregulation (3) ; or(b) including, in an authorisation under subregulation (1) , a printed label identifying the patient if that label is initialled by the medical practitioner, dentist or authorised nurse practitioner.(3) A medical practitioner, dentist or authorised nurse practitioner who verbally authorises the emergency administration of a narcotic substance to a patient under subregulation (2)(a) must, within 24 hours after giving those instructions, sign an entry in the patient's medical history clearly indicating that the medical practitioner, dentist or authorised nurse practitioner authorised the administration of that substance.(4) If of the opinion that it is necessary for a patient's wellbeing, a registered nurse may continue to administer a narcotic substance to that patient in accordance with a verbal authorisation under subregulation (3) even though the medical practitioner, dentist or authorised nurse practitioner has not signed an entry in accordance with that subregulation.(5) A person must not administer a narcotic substance to a patient in a medical institution except –(a) in a case to which subregulation (2)(a) or subregulation (4) applies; or(b) as otherwise provided in the Act or these regulations; or(c) on the written authorisation of a medical practitioner, dentist or authorised nurse practitioner.Penalty: Fine not exceeding 10 penalty units.
Division 9 - Storage and use of narcotic substances in special cases
31. Minister may grant nurse's authority in certain circumstances
(1) Where, on the application of a registered nurse, the Minister is satisfied that the nurse carries on his or her profession under such circumstances that the services of a medical practitioner are not readily available to give instructions for the administration of narcotic substances to the patients upon whom the nurse attends, the Minister may grant the nurse a nurse's authority.(2) A nurse's authority granted under subregulation (1) –(a) authorises its holder to have possession of such narcotic substances for the purpose for which the authority is issued and in such quantities as may be specified in the authority; and(b) ceases to have effect when the person by whom it is held ceases to hold the appointment or employment specified in the authority.(3) On an order in writing signed by the holder of a nurse's authority granted under subregulation (1) and upon production by the holder of the authority, a pharmaceutical chemist may supply the holder of the authority with such narcotic substances and in such quantities as may be specified in the authority.(4) A person who has been granted a nurse's authority under this regulation must keep a narcotic substances register in accordance with regulation 13 .Penalty: Fine not exceeding 10 penalty units.
32. Narcotic substances on vessels
(1) The master of a vessel may have possession of such narcotic substances as may be necessary for the purpose of ensuring that the requirements of the law of the Commonwealth or of the Marine and Safety Authority Act 1997 are complied with in respect of the vessel, if those substances have been supplied to the master on an order in writing signed by the master.(2) Without prejudice to the operation of subregulation (1) , a master of a vessel may keep on that vessel any narcotic substance that the Secretary may, in writing, allow to be kept on the vessel so long as –(a) the amount so kept does not exceed the amount of that substance that the Secretary has allowed to be so kept; and(b) any requirements made by the Secretary in respect of the place or manner in which the substance is kept on that vessel and in respect of the keeping of records of the acquisition, use or disposal of the substance are complied with.(3) A chemist may supply a narcotic substance to the master of a vessel on an order in writing signed by the master, if the chemist is satisfied that the substance is required for the purpose referred to in subregulation (1) or that the keeping of that substance on the vessel is allowed under subregulation (2) .(4) In this regulation –master includes the person (not being a pilot or harbourmaster) for the time being in charge or having control of any vessel.
33. Narcotic substances for first aid use
(1) A person authorised under regulation 92 to possess a narcotic substance must enter or cause to be entered in a register kept solely for that purpose a record of –(a) all supplies of any narcotic substances procured or which otherwise come into the possession of that person; and(b) the quantity of the narcotic substance stored in approved first aid kits by that person together with the information as to the place in which the narcotic substance is so stored; and(c) the date on which and the place in which the narcotic substance is used and the quantity of the narcotic substance that is used.Penalty: Fine not exceeding 10 penalty units.(2) Each entry made in the register is to –(a) indicate the amount of each narcotic substance to which the entry relates that is stored in the approved first aid kit immediately after completion of the event to which the entry relates; and(b) be signed by the person by whom the entry is made.(3) A person who keeps a narcotic substance in an approved first aid kit must, as soon as possible after the narcotic substance has been used, notify the Secretary of that use.Penalty: Fine not exceeding 10 penalty units.
Division 10 - Destruction, disposal and loss, &c., of narcotic substances
34. Destruction of narcotic substances prohibited
(1) A person who is licensed or authorised to be in possession of a narcotic substance must not wilfully –(a) destroy that narcotic substance; or(b) cause or permit that narcotic substance to be destroyed.Penalty: Fine not exceeding 10 penalty units.(2) Subregulation (1) does not apply to the destruction of a narcotic substance –(a) by or under the personal supervision of an inspector under a direction of the Minister; or(b) in the possession of a person obtained pursuant to the prescription of a medical practitioner, veterinary surgeon or dentist; or(c) by the person for the time being holding, or performing the duties of, the office of Chief Pharmacist in the Department in the course of the duties of that office; or(d) by the holder of an office of analyst under section 19 of the Act in the course of the duties of that office; or(e) by a person authorised in writing to do so by the Secretary; or(f) by any 2 health professionals working jointly to destroy the narcotic substance; or(g) by an enrolled nurse working jointly with a health professional to destroy the narcotic substance.(3) An authorisation given under subregulation (2)(e) is subject to any conditions specified in it.(4) A person who destroys, or works jointly with another person to destroy, a narcotic substance must complete a narcotic substances register in accordance with regulation 13 in respect of that substance.Penalty: Fine not exceeding 10 penalty units.
35. Procedures in case of loss, &c., of narcotic substances
(1) In this regulation –(a) a reference to the unintentional destruction of a narcotic substance includes a reference to the unintentional disposal of that substance; and(b) a reference to a narcotic substance includes a reference to a narcotic substance in a parenteral solution.(2) If a narcotic substance is lost in a medical institution, clause 1 of Schedule 8 has effect.(3) If a narcotic substance is spilt, broken or unintentionally destroyed in a medical institution, clause 2 of Schedule 8 has effect.
Division 11 - General
36. Miscellaneous duties of licence holder
(1) The holder of a licence under this Part –(a) must not sell, keep or otherwise have possession of a narcotic substance except upon the premises specified in the licence; and(b) must not transact any dealing in a narcotic substance except by himself or herself or by a competent or responsible person acting on his or her behalf; and(c) must not sell or supply a narcotic substance to any person who is not authorised under these regulations to purchase or receive the substance; and(d) must keep and store a narcotic substance in his or her possession in accordance with these regulations; and(e) must keep and maintain a narcotic substances register in accordance with these regulations.Penalty: Fine not exceeding 10 penalty units.(2) Notwithstanding subregulation (1)(a) , the Minister may authorise, in writing, a holder of a licence to have possession of a narcotic substance for such purpose and for such period as may be specified in the authority.
37. Self-administration of narcotic substances prohibited
(1) A person must not administer or cause to be administered to himself or herself by any means whatsoever a narcotic substance that is in the person's possession, unless the narcotic substance has been supplied to the person for that purpose –(a) by, or on a prescription issued by, a medical practitioner or authorised nurse practitioner; or(b) by an authorised nurse; or(c) by a dentist; or(d) by a person on the staff of a hospital in accordance with the instructions of a medical practitioner.Penalty: Fine not exceeding 10 penalty units.(2) Nothing in this regulation prohibits the administration to a person of a narcotic substance required to be kept in any aircraft or vessel in the case of an emergency where the services of a medical practitioner are not readily available.
38. Control of narcotic substances within Ambulance Service
(1) Subject to subregulation (2) , the Director of Ambulance Services may issue directives to ambulance officers in respect of the following matters:(a) the procurement and distribution of narcotic substances for use by the Ambulance Service;(b) the keeping of records and the furnishing of reports and returns by ambulance officers in respect of narcotic substances;(c) the procedures to be followed, and the precautions to be taken, by ambulance officers in respect of –(i) the storage, handling and use of narcotic substances, whether in ambulance vehicles or elsewhere; and(ii) the loss, spillage, breakage or unintentional destruction of narcotic substances; and(iii) the disposal of unused or out-of-date narcotic substances.(2) A directive issued under subregulation (1) –(a) is not to contain a provision that is inconsistent with, or contrary to, a provision of the Act or these regulations; and(b) is of no effect until it has been approved by the Secretary.
PART 4 - Poisons and Restricted Substances
Division 1 - Storage of poisons, &c.
(1) Where, at any premises, a person has possession, custody or control of, for sale or supply, a substance specified in Schedule 7 of the Poisons List, the person must keep the substance in a part of the premises that is partitioned off or otherwise separated from any part of the premises that is readily accessible to the public.Penalty: Fine not exceeding 10 penalty units.(2) A chemist, medical practitioner, dentist, authorised nurse practitioner, veterinary surgeon or optometrist who sells or supplies any substance specified in Schedule 3 or 4 to the Poisons List must keep it in either of the following so that the public does not have access to the substance:(a) a storeroom; or(b) the dispensary.Penalty: Fine not exceeding 10 penalty units.(3) If a person has possession, custody or control of, for sale or supply, a substance specified in Schedule 2 of the Poisons List –(a) at premises which are not a pharmacy, the person must keep the substance behind a serving counter or in such other manner as to ensure that it is not readily accessible to the public; or(b) at a pharmacy, the person must keep the substance as specified in paragraph (a) or on a horizontal shelf that is –(i) affixed to, or placed immediately against, an internal wall or partition separating the dispensary from the remainder of the premises; or(ii) not more than 4 metres from, and in clear line of sight of, the dispensary; or(iii) susceptible, in such other manner as may be approved by the Secretary, of close supervision from the dispensary.Penalty: Fine not exceeding 10 penalty units.(4) Where a person has possession, custody or control of, for sale or supply, a poison, the person must keep it separate and apart from other goods suitable for human or animal consumption in such a way that, if the container of the poison breaks or leaks, the poison will not intermix with or contaminate those goods.Penalty: Fine not exceeding 10 penalty units.
Division 2 - Advertising, prescribing, sale, supply and dispensing of restricted substances
40. Restriction on advertising of restricted substances
A person must not advertise a restricted substance except in publications that circulate generally only among persons lawfully engaged in medical, dental, nurse practitioner, veterinary, optometrical or pharmaceutical practice or in the manufacture or supply of restricted substances.Penalty: Fine not exceeding 10 penalty units.
41. Prescriptions for restricted substances
(1) A person, other than a medical practitioner, dentist, authorised nurse practitioner, veterinary surgeon or authorised optometrist must not issue a prescription for a restricted substance.Penalty: Fine not exceeding 10 penalty units.(2) A medical practitioner, dentist or veterinary surgeon, subject to this regulation, is authorised to issue a prescription for any restricted substance.(3) An authorised nurse practitioner may write or issue a prescription for a restricted substance in such circumstances, subject to such conditions and in relation to such substances or classes of substances as may be specified in an authorisation issued by the Secretary.(4) Subject to regulation 42 , a medical practitioner, dentist, authorised nurse practitioner, veterinary surgeon or authorised optometrist on issuing a prescription must comply with the following conditions:(a) except in a case where the Secretary otherwise approves, he or she is to write the prescription legibly in ink and, in his or her own handwriting, is to include in the prescription –(i) the date on which it is written; and(ii) the name and address of the patient or, in the case of an animal, the name and address of its owner; and(iii) the name and the quantity of the restricted substance to be dispensed; and(iv) adequate directions for use; and(v) the number of times (if any) the dispensing of the prescription may be repeated; and(vi) where the prescription is for a declared restricted substance and the dispensing of the prescription is authorised, by its terms, to be repeated, the minimum intervals at which it may be dispensed;(b) he or she must sign the prescription;(c) the prescription must include –(i) in print, the name and address of the medical practitioner, dentist, authorised nurse practitioner, veterinary surgeon or authorised optometrist; or(ii) in print or block letters, the name and address of the person writing or issuing the prescription, where the patient is attending a public hospital; or(iii) in print or block letters, the name of the person writing or issuing the prescription where the patient is attending a public hospital and the address of that hospital appears on the prescription;(d) the prescription must be issued –(i) by a medical practitioner for use in the course of medical treatment only; or(ii) by a dentist for use in the course of dental treatment only and is to include the words "For dental treatment only"; or(iii) by an authorised nurse practitioner for use in the course of nurse practitioner treatment only; or(iv) by a veterinary surgeon for use in the course of animal treatment only and is to include the words "For animal treatment only"; or(v) by an authorised optometrist, for use in the course of optometrical treatment only;(e) where the prescription contains an unusual dose, the medical practitioner, dentist, authorised nurse practitioner, veterinary surgeon or authorised optometrist by whom it is issued must underline, or by some other means emphasise, that part of the prescription that refers to the unusual dose and, except for a prescription issued in accordance with an approval under regulation 42 , initial that part in the margin.Penalty: Fine not exceeding 10 penalty units.(5) Notwithstanding subregulation (4)(a)(ii) , a medical practitioner, dentist, authorised nurse practitioner, veterinary surgeon or authorised optometrist may use a printed label in a prescription to identify the patient or owner of the animal, if that label is initialled by the medical practitioner, dentist, authorised nurse practitioner, veterinary surgeon or authorised optometrist.(6) A prescription is to be taken, for the purposes of these regulations, to have been issued in accordance with subregulation (4) where –(a) the address of the patient, as shown on the prescription for a restricted substance issued by a medical practitioner, authorised nurse practitioner or authorised optometrist, is a medical institution; and(b) in place of adequate directions for use, the prescription includes a notation to the effect that the restricted substance is to be used as directed.(7) A medical practitioner, authorised nurse practitioner or authorised optometrist must not issue a prescription for a restricted substance in the form referred to in subregulation (6) unless, at the time of issue of the prescription –(a) the patient to whom the prescription relates is a patient in the medical institution referred to in the prescription; and(b) adequate directions, in writing, for the use of the restricted substance in relation to the patient have been, or are, given by the medical practitioner, authorised nurse practitioner or authorised optometrist to a person having responsibility for the care of the patient in the medical institution.Penalty: Fine not exceeding 10 penalty units.
42. Electronic prescriptions for restricted substances
(1) Where a medical practitioner, dentist, authorised nurse practitioner, veterinary surgeon or authorised optometrist is authorised to issue a prescription for a restricted substance, that prescription may be issued electronically if the Secretary so approves.(2) For the purpose of subregulation (1) , issuing a prescription electronically includes issuing the prescription by transmitting data electronically without producing a printed copy of that data.(3) An approval under subregulation (1) –(a) is to be in writing signed by the Secretary; and(b) may be of general or specific application; and(c) may be made subject to such conditions as the Secretary thinks fit; and(d) may be amended or revoked by the Secretary by notice in writing.(4) The conditions specified in regulation 41(4) , with the exception of the condition that a prescription be handwritten in ink, apply to the issuing of a prescription electronically in accordance with an approval under subregulation (1) .(5) For the purposes of regulation 41(4)(b) as it applies to the issuing of a prescription in accordance with an approval under subregulation (1) , sign includes sign electronically.
43. Emergency prescribing and dispensing of restricted substances
(1) Notwithstanding regulation 41(4) , a pharmaceutical chemist may supply a restricted substance on the instruction of a medical practitioner, veterinary surgeon, dentist, authorised nurse practitioner or authorised optometrist if, because of the urgent circumstances in which the substance is required, it is impracticable, before the substance is required to be supplied, to –(a) issue a prescription for the substance; and(b) cause the prescription to be delivered to the chemist.(2) A medical practitioner, veterinary surgeon, dentist, authorised nurse practitioner or authorised optometrist who issues an instruction under subregulation (1) must send to that chemist, within 24 hours of issuing the instruction, a prescription that –(a) complies with regulation 41(4) ; and(b) clearly states that it is in confirmation of the instruction to that chemist to supply the restricted substance without a prescription.Penalty: Fine not exceeding 10 penalty units.
44. Records of prescribing of declared restricted substances
(1) As soon as practicable after a medical practitioner, dentist, authorised nurse practitioner or veterinary surgeon issues a prescription for a declared restricted substance, he or she must make a record of that prescription, in a form approved by the Secretary, showing –(a) the name and address of the person for the treatment of whom, or of the owner of the animal for the treatment of which, the substance was prescribed; and(b) the date of issue of the prescription; and(c) particulars of the substance sufficient to identify it and to indicate in what quantity and strength it was prescribed; and(d) particulars of the directions set out in the prescription for the use of the substance; and(e) particulars of any provision made in the prescription in respect of the number of times, and the minimum intervals at which, the dispensing of the prescription was authorised to be repeated.Penalty: Fine not exceeding 10 penalty units.(2) A medical practitioner, dentist, authorised nurse practitioner or veterinary surgeon must retain a record made under subregulation (1) for not less than 2 years.Penalty: Fine not exceeding 10 penalty units.
45. Sale or supply of restricted substances
(1) Where any medical practitioner, dentist, authorised nurse practitioner, veterinary surgeon or authorised optometrist sells or supplies a restricted substance otherwise than by way of wholesale dealing, the substance must be sold or supplied –(a) by the medical practitioner only for use in the course of medical treatment; or(b) by the dentist only for use in the course of dental treatment; or(c) by the authorised nurse practitioner only for use in the course of nurse practitioner treatment; or(d) by the veterinary surgeon only for use in the course of animal treatment; or(e) by the authorised optometrist only for use in the course of optometrical treatment.(2) Where any medical practitioner, dentist, authorised nurse practitioner, veterinary surgeon or optometrist sells or supplies a restricted substance other than by way of wholesale dealing in a quantity exceeding that required for 3 days' treatment, he or she must comply with the following conditions:(a) before the restricted substance is sold or supplied a record of the sale or supply of that substance is to be made showing –(i) the date on which it was supplied; and(ii) the name and address of the person for whose treatment it was supplied or in the case of an animal the name and address of the owner; and(iii) the name and quantity of the restricted substance supplied;(b) the label on the container is to bear the particulars prescribed in regulation 84 ;(c) the record of the supply of the restricted substance is to be kept at the surgery or office of the person by whom that substance was supplied or sold, and is to be produced on demand to an inspector.Penalty: Fine not exceeding 10 penalty units.
46. Dispensing prescriptions for restricted substances (S4)
(1) Subject to subregulations (2) and (3) and to regulation 48 , a person must not supply a restricted substance otherwise than on, and in accordance with, a prescription issued, or a direction given, in accordance with regulation 41 .Penalty: Fine not exceeding 10 penalty units.(2) A person may dispense not more than once a prescription for a restricted substance that conforms in all other respects with regulation 41(4) but does not specify a maximum number of times the dispensing of the prescription may be repeated if, upon dispensing the prescription, that person cancels it in accordance with subregulation (12) .(3) Where –(a) the dispensing of a prescription for a declared restricted substance is authorised, by the terms of the prescription, to be repeated but the prescription does not specify the minimum intervals at which the dispensing of the prescription may be repeated; and(b) the prescription conforms in all other respects with regulation 41(4) –a person may dispense that prescription if –(c) the person –(i) ascertains, in accordance with subregulation (4) , the earliest date on which the dispensing of the prescription may be repeated; and(ii) upon dispensing the prescription, marks on it, over the person's signature and the date, a note that specifies both the date so ascertained and how it was ascertained; or(d) upon dispensing the prescription, the person cancels it in accordance with subregulation (12) .(4) The date referred to in subregulation (3)(c)(i) is to be ascertained by reference to either or both of the following:(a) specific directions for the use by the patient of the declared restricted substance, being directions incorporating a dosage rate, that are included in the prescription;(b) information obtained directly from the prescriber as to the minimum interval between occasions on which the declared restricted substance should be dispensed to the patient.(5) If the date referred to in subregulation (3)(c)(i) is ascertained by reference only to the information referred to in paragraph (a) of subregulation (4) , that date is to be a date not earlier than 4 days before the day on which the quantity of the declared restricted substance being dispensed would be exhausted if used strictly in accordance with the directions referred to in that paragraph.(6) A person, other than a pharmaceutical chemist or an assistant under the direct personal supervision of a pharmaceutical chemist, must not dispense a prescription for a restricted substance.Penalty: Fine not exceeding 10 penalty units.(7) A pharmaceutical chemist or an assistant under the direct personal supervision of a pharmaceutical chemist, subject to this regulation, is authorised to dispense a prescription for a restricted substance.(8) A person must not dispense any prescription for a restricted substance or supply a restricted substance on or in accordance with any prescription unless the person complies with the following provisions:(a) the dispensing of the prescription must not be repeated more than the maximum number of times indicated on it and, if an interval of times is specified, at a shorter interval than that specified, and on each occasion upon which it is dispensed there is to be stamped or marked in writing or clearly shown on the prescription –(i) the date upon which it is dispensed; and(ii) the signature of the pharmaceutical chemist by whom it is dispensed or by whom its dispensing is supervised; and(iii) the address of the place at which it is dispensed;(b) where the occasion of the dispensing of the prescription is, in accordance with the terms of the prescription, the last occasion on which it may be dispensed, the person, upon dispensing it, must cancel it in accordance with subregulation (12) ;(c) before completion of the supply of the restricted substance, the person must make or cause to be made, in the approved recording system relating to the pharmacy at which that supply is effected, an entry that, subject to subregulation (9) , sets out –(i) the date on which the restricted substance is dispensed; and(ii) whether the authority for the dispensing by the person of the restricted substance is a prescription issued under regulation 41 or 42 or an instruction given under regulation 43 ; and(iii) the name of the person by whom that prescription was issued or that direction was given; and(iv) the name and address of the person for the treatment of whom, or of the owner of the animal for the treatment of which, the restricted substance was dispensed; and(v) the material part of the prescription or instruction, including particulars of any specification in respect of the repeated dispensing of the prescription; and(vi) the text of any note marked, under subregulation (3)(c)(ii) , on the prescription (if any) on the authority of which that person dispensed the restricted substance;(d) the label on the container of the restricted substance is to be marked with the identifying letter or number of the prescription appearing in the approved recording system and with such other particulars as are prescribed in regulation 84(2) ;(e) the approved recording system is to be kept at the place at which the restricted substance was dispensed and is produced on demand to an inspector.Penalty: Fine not exceeding 10 penalty units.(9) Where the dispensing of a prescription is repeated at a pharmacy at which it was previously dispensed, the entry made under subregulation (8)(c) in relation to the repeat is to set out –(a) the date of the repeat; and(b) the name and address of the person for the treatment of whom, or of the owner of the animal for the treatment of which, the dispensing of the prescription was repeated; and(c) a note of the fact of the repeat by reference to the entry made under subregulation (8)(c) in relation to the first occasion of the dispensing of that prescription in that pharmacy.(10) As soon as possible after a prescription written under regulation 43 is received at the pharmacy at which the restricted substance to which it relates was dispensed, a pharmaceutical chemist engaged in the conduct of that pharmacy must –(a) clearly mark on the prescription a note setting out –(i) the date on which the restricted substance to which it relates was dispensed; and(ii) the name of the pharmaceutical chemist by whom, or under whose supervision, it was dispensed; and(iii) the name and address of the pharmacy at which it was dispensed; and(iv) the identifying mark relating to the approved recording system entry made in pursuance of subregulation (8)(c) in relation to the dispensing of that restricted substance; and(b) sign and date that note.Penalty: Fine not exceeding 10 penalty units.(11) Where the prescription is such that, if it were given otherwise than under regulation 43 , subregulation (2) or (3) of this regulation would apply in relation to it, the pharmaceutical chemist must cancel it, or mark it, as required by whichever of those subregulations would so apply.Penalty: Fine not exceeding 10 penalty units.(12) Subject to subregulations (13) and (14) , where a person is required by any of the preceding provisions of this regulation to cancel a prescription –(a) the person must write, stamp or mark in ink in legible letters the word "Cancelled" across the prescription; and(b) except with the permission in writing of the Secretary, the person must not, within a period of 2 years from the day on which the requirement to cancel the prescription arose, remove or permit the removal of the prescription from the premises at which that requirement arose.Penalty: Fine not exceeding 10 penalty units.(13) Where the original of a prescription that is prepared in duplicate is required, under a law of the Commonwealth, to be forwarded to a Department or authority of the Commonwealth –(a) it is sufficient compliance with the requirements of subregulation (3)(c)(ii) , subregulation (8)(a) , subregulation (10) or (12) if the duplicate of the prescription is dealt with in accordance with those requirements; and(b) a reference in the following provisions of this regulation to a prescription, unless the contrary intention appears, is to be taken to include a reference to such a duplicate.(14) Subregulation (12)(b) does not apply in relation to a prescription (other than a prescription that does not comply with regulation 15 ) that is written on the same piece of paper as another prescription that may lawfully subsequently be dispensed.(15) A person must not dispense a restricted substance on a prescription which –(a) is illegible or defaced; or(b) is marked "cancelled"; or(c) appears to have been altered; or(d) appears to be fraudulent in any respect or forged.Penalty: Fine not exceeding 10 penalty units.(16) A person to whom a prescription of the kind referred to in subregulation (15) is presented must –(a) retain the prescription notwithstanding that it has not been dispensed; and(b) as soon as practicable inform the Secretary or a police officer of the relevant circumstances and the person's reasons for not dispensing the prescription.Penalty: Fine not exceeding 10 penalty units.(17) A person must not dispense a prescription for a restricted substance or supply a restricted substance upon a prescription if the prescription is presented for dispensing –(a) in the case of a prescription for a restricted substance other than a declared restricted substance or a specified psychotropic substance, more than 12 months after the date on which the prescription was written; or(b) in the case of a prescription for a declared restricted substance or a specified psychotropic substance, more than 6 months after the date on which the prescription was written.Penalty: Fine not exceeding 10 penalty units.(18) For the purposes of this regulation, 2 or more prescriptions issued to the same person in respect of the same declared restricted substance are to be treated as a single prescription that authorises the substance to be dispensed more than once.
47. Specified psychotropic substances
The restricted substances specified in Schedule 4 are specified psychotropic substances for the purposes of section 38(1)(b) of the Act.
48. Emergency supply of restricted substances other than specified psychotropic substances
The following conditions are prescribed, for the purposes of section 38(1)(b) of the Act, in relation to the sale or supply by a pharmaceutical chemist, otherwise than on and in accordance with a prescription of a medical practitioner, dentist, authorised nurse practitioner or veterinary surgeon, of a restricted substance other than a specified psychotropic substance:(a) the pharmaceutical chemist must, before supplying the restricted substance, be satisfied, on reasonable grounds, that –(i) the person for the treatment of whom the supply of the restricted substance is sought ("the patient") is undergoing medical treatment which requires the use of that restricted substance; and(ii) the continuation of that treatment is essential to the wellbeing of the patient; and(iii) it is not practicable for the patient to obtain a prescription for that restricted substance before the supply of it for his or her treatment will be necessary;(b) the quantity of the restricted substance supplied must not exceed –the minimum quantity that may be dispensed without interfering with the integrity of that packaging and is not less than the quantity referred to in subparagraph (i) ;(i) the quantity necessary for 3 days' treatment of the patient; or(ii) in the case of a restricted substance that is in the form of –(A) a cream or ointment supplied to the pharmaceutical chemist in a form of packaging designed for supply intact to individual users; or(B) a liquid supplied to the pharmaceutical chemist in a form of packaging designed for supply intact to individual users, being a metered-dose pressurised spray pack, a dropper or applicator bottle or a form of packaging interference with the integrity of which would be detrimental to the proper administration to the patient of the restricted substance –(c) the container in which the restricted substance is supplied must be labelled in accordance with regulation 87(2) and (3) and, in addition, the principal label on that container must include –(i) the words "EMERGENCY SUPPLY"; and(ii) the date of supply; and(iii) the identifying mark relating to the entry made under paragraph (d) in relation to the supply of that restricted substance;(d) before completion of the supply of the restricted substance, the pharmaceutical chemist must make, in the approved recording system relating to the pharmacy at which that supply is effected, an entry signed by that pharmaceutical chemist that sets out –(i) the date on which the restricted substance is supplied; and(ii) a note to the effect that the supply of the restricted substance was by way of emergency supply under this regulation, including the reasons for that emergency supply and, to the best of the knowledge and belief of the pharmaceutical chemist, the name of the medical practitioner or authorised nurse practitioner by whom the restricted substance was last prescribed for the patient; and(iii) the name and address of the patient; and(iv) particulars of the restricted substance sufficient to identify it and to indicate in what quantity and strength it was supplied; and(v) the directions given for the use by the patient of the restricted substance.
49. Supply of restricted substance in medical institution
(1) For the purposes of section 38(1)(b) of the Act, a pharmaceutical chemist may sell or supply a restricted substance to a resident of, or a patient in, a medical institution, otherwise than in accordance with a prescription of a medical practitioner, dentist or authorised nurse practitioner, if –(a) a valid prescription does not exist; and(b) the substance is included on the drug therapy chart of the resident or patient and the chemist has seen the chart or a copy of it; and(c) the chemist is satisfied that the sale or supply of the substance is necessary for the continued treatment of the resident or patient; and(d) the chemist makes a record in an approved recording system of –(i) the name of the medical practitioner, dentist or authorised nurse practitioner responsible for preparing the drug therapy chart and the person to whom it is sold or supplied; and(ii) the name and amount of the substance sold or supplied; and(iii) the basis on which the substance is sold or supplied; and(e) the amount of the substance supplied does not exceed the smallest practicable amount and in any case does not exceed –(i) if the substance is included in the Schedule of Pharmaceutical Benefits for Approved Pharmacists and Medical Practitioners published under Part VI of the National Health Act 1953 of the Commonwealth, the maximum quantity specified in that Schedule; or(ii) if the substance is not included in that Schedule, one month’s supply.(2) Only one sale or supply of the substance may be made to the resident or patient under subregulation (1) .(3) If a restricted substance is supplied under this regulation, the medical practitioner or authorised nurse practitioner responsible for preparing the drug therapy chart must send to the pharmaceutical chemist referred to in subregulation (1) , within 24 hours after the supply, a prescription that –(a) complies with regulation 41(4) ; and(b) clearly states that it is in authorisation of the supply, otherwise than in accordance with a prescription, by that chemist of the restricted substance.
(1) Subject to subregulation (2) , a pharmaceutical chemist, medical practitioner, dentist or authorised nurse practitioner must not supply to a person a substance listed in Schedule 5 unless, at the time that he or she supplies the substance, he or she also supplies to the person to whom the substance is supplied an information sheet, in a form approved by the Secretary, concerning that substance.(2) An information sheet need not be supplied under subregulation (1) if –(a) the substance is supplied by a pharmaceutical chemist and the prescription according to which it is dispensed bears an endorsement that it is not necessary for the pharmaceutical chemist to supply the information sheet; or(b) in a case where a medical practitioner, dentist or authorised nurse practitioner supplies the substance without the intervention of a pharmaceutical chemist, the medical practitioner, dentist or authorised nurse practitioner is of the opinion that it is not in the patient's interests to supply the sheet.
Division 3 - Advertising, sale, supply, dispensing and recording of potent substances
51. Interpretation of Division
For the purposes of this Division –school means a school within the meaning of the Education Act 1994 ;specified potent substance means a potent substance that is specified in Schedule 6 .
52. Advertising of potent substances
A person must not advertise a potent substance except –(a) in a publication circulating generally only among persons lawfully engaged in –(i) medical, dental, veterinary, nurse practitioner or pharmaceutical practice; or(ii) the manufacture or supply of potent substances; or(b) in accordance with the Uniform Standard.Penalty: Fine not exceeding 10 penalty units.
53. Supply of potent substances by pharmaceutical chemists
(1) A pharmaceutical chemist must not supply a potent substance unless –(a) the supply is made on the authority of a medical practitioner, veterinary surgeon, dentist or authorised nurse practitioner, whether given in the form of a prescription or otherwise; or(b) the pharmaceutical chemist, or a pharmaceutical chemist or trainee pharmacist employed by that pharmaceutical chemist –(i) participates personally and directly in the supply of the substance; and(ii) on consideration of the condition, disease or symptoms of the person for whom, or the animal for which, the substance is supplied (in this regulation referred to as "the patient") forms the opinion that the use of that substance in the treatment of the patient is justified; or(c) the supply is made for a school first aid kit in accordance with the written authority of the school principal; or(d) the supply is made to a person or class of persons approved by the Secretary as having sufficient expertise to administer the substance.Penalty: Fine not exceeding 10 penalty units.(2) A pharmaceutical chemist must not supply a Schedule 3 substance that is a specified potent substance, other than salbutamol (S3) supplied for a school first aid kit in accordance with the written authority of the school principal, unless its container is labelled in accordance with regulation 84(2) and (3) .Penalty: Fine not exceeding 10 penalty units.(3) A pharmaceutical chemist must not supply salbutamol (S3) for a school first aid kit otherwise than in accordance with subregulation (4)(b) .Penalty: Fine not exceeding 10 penalty units.(4) A pharmaceutical chemist must not supply a Schedule 3 substance that is not a specified potent substance unless –(a) its container is labelled in accordance with regulation 84(2) and (3) ; or(b) in addition to its container and any primary pack conforming to the requirements applicable under Part 5 –(i) its container bears a label that identifies the pharmacy from which it was supplied; and(ii) there are supplied with it directions for its use as specified in subregulation (5) .Penalty: Fine not exceeding 10 penalty units.(5) The directions referred to in subregulation (4)(b)(ii) for the use of a substance are to comprise either –(a) adequate written directions, on a label on its container, for its use specifically in the treatment of the patient; or(b) both of the following:(i) written directions, on a label on its container, for its use generally;(ii) an oral explanation of the specific application of those directions in relation to the treatment of the patient, being an explanation given by a pharmaceutical chemist or a pharmacy trainee to the patient or, where the potent substance is to be administered to the patient by another person, to that other person.
54. Administration of salbutamol (S3)
(1) A person employed by a school may administer to a child salbutamol (S3) in accordance with its directions for use.(2) As soon as possible after a person administers salbutamol (S3) to a child, the person is to advise the principal of the school of the following:(a) the name of the person;(b) the name of the child;(c) the amount of salbutamol (S3) administered;(d) the date on which the salbutamol (S3) was administered;(e) any other relevant information.(3) The principal of a school is responsible for the storage, and control of the use, of any salbutamol (S3) kept by the school.(4) The following people may administer to another person salbutamol (S3) in accordance with its directions for use:(a) a St John Ambulance officer;(b) the holder of a current relevant certificate issued on behalf of the Asthma Foundation of Tasmania;(c) any other person the Secretary is satisfied has appropriate training or qualifications to administer salbutamol (S3).
55. Obtaining potent substances through misrepresentation
A person must not make a representation, whether oral or in writing, which the person knows to be false or misleading, or engage in conduct which the person knows to be deceptive, for the purpose, whether achieved or not, of –(a) obtaining the authority of a medical practitioner, dentist, authorised nurse practitioner or veterinary surgeon, whether in the form of a prescription or otherwise, for the supply of a potent substance; or(b) inducing a medical practitioner, dentist, authorised nurse practitioner, veterinary surgeon or pharmaceutical chemist to supply a potent substance.Penalty: Fine not exceeding 10 penalty units.
Division 4 - Use, &c., of restricted substances in medical institutions
56. Authorised officer to store, &c., restricted substances
(1) In a medical institution the authorised officer must –(a) receive all restricted substances supplied to that institution for the purposes of that institution; and(b) store those restricted substances until their supply in accordance with subregulation (2) ; and(c) keep such records of those restricted substances as are required by these regulations.Penalty: Fine not exceeding 10 penalty units.(2) A person other than the authorised officer must not supply restricted substances received and stored in accordance with subregulation (1) , and the authorised officer must not supply a restricted substance, except –(a) on a prescription written in accordance with regulation 41 ; or(b) in the case of the supply of a restricted substance to a ward, on an order in writing of –(i) the registered nurse in charge of the ward in which the restricted substance is to be used or stored; or(ii) a medical practitioner, dentist or authorised nurse practitioner.Penalty: Fine not exceeding 10 penalty units.
57. Supply of restricted substances to patients
(1) Where any person authorised in accordance with the Act supplies a restricted substance to a patient in a medical institution, the person must do so in the original container in which the restricted substance was received from the manufacturer or distributor.Penalty: Fine not exceeding 10 penalty units.(2) Subregulation (1) does not apply –(a) to the supply of an individual dose to a patient in that institution; or(b) to the supply of a restricted substance packed and labelled under the direct personal supervision of a medical practitioner, pharmaceutical chemist, dentist or authorised nurse practitioner.
58. Storage of declared restricted substances in wards of medical institutions
The registered nurse in charge of a ward of a medical institution must –(a) keep the declared restricted substances supplied to that ward stored apart from all other goods, other than narcotic substances, in a separate cupboard or receptacle that is securely fixed to the premises; and(b) keep that cupboard or receptacle securely locked at all times when the substances in it are not being used.Penalty: Fine not exceeding 10 penalty units.
59. Administration of restricted substances (S4) in medical institutions
(1) Subject to subregulation (2) and regulation 94 , a medical practitioner, dentist or authorised nurse practitioner must not give instructions for a restricted substance to be administered to a patient in a medical institution without completing and signing, in his or her own handwriting or in a manner approved by the Secretary, an authorisation to do so.Penalty: Fine not exceeding 10 penalty units.(2) Nothing in subregulation (1) prohibits a medical practitioner, dentist or authorised nurse practitioner from –(a) giving oral instructions for a restricted substance to be administered to a patient in a medical institution in an emergency if the medical practitioner, dentist or authorised nurse practitioner subsequently complies with subregulation (3) ; or(b) including, in an authorisation under subregulation (1) , a printed label identifying the patient if that label is initialled by the medical practitioner, dentist or authorised nurse practitioner.(3) A medical practitioner, dentist or authorised nurse practitioner who orally authorises the emergency administration of a restricted substance to a patient under subregulation (2)(a) must, within 24 hours after giving those instructions, sign an entry in the patient's medical history clearly indicating that the medical practitioner, dentist or authorised nurse practitioner authorised the administration of that substance.Penalty: Fine not exceeding 10 penalty units.(4) If of the opinion that it is necessary for a patient's wellbeing, a registered nurse may continue to administer a restricted substance to that patient in accordance with a verbal authorisation under subregulation (3) even though the medical practitioner, dentist or authorised nurse practitioner has not signed an entry in accordance with that subregulation.(5) A person must not administer a restricted substance to a patient in a medical institution except –(a) in a case to which subregulation (2)(a) or subregulation (4) applies; or(b) as otherwise provided in the Act or these regulations; or(c) on the written authorisation of a medical practitioner, dentist or authorised nurse practitioner.Penalty: Fine not exceeding 10 penalty units.
Division 5 - Administration of substances by nurses
60. Enrolled nurse may administer certain substances
For the purposes of section 38(1)(i) of the Act, an enrolled nurse may administer a substance listed in Schedule 2 , 3 , 4 or 8 to the Poisons List if the enrolled nurse –(a) acts –(i) in accordance with the written authority of a medical practitioner, dentist or authorised nurse practitioner; and(ii) under the supervision of a medical practitioner, dentist, authorised nurse practitioner or registered nurse; and(b) holds any qualifications that the Executive Officer of the Nursing Board of Tasmania determines are appropriate for the administration of that substance.
61. Registered nurse may possess and administer restricted substance
A registered nurse may possess and administer to a person a restricted substance without instructions from a doctor if –(a) the nurse is attending an emergency in a remote area and the person requires urgent treatment with medication; and(b) it is not practicable to obtain instructions from a doctor; and(c) the nurse has –(i) undergone an educational program approved by the Nursing Board of Tasmania; and(ii) been authorised by that Board; and(d) the nurse follows appropriate procedures approved by the Secretary.
62. Minister’s authorisation for possession and supply of restricted substances
The Minister may make an authorisation under section 25A of the Act in respect of a restricted substance, or a class of restricted substances, in the following circumstances:(a) where the registered nurse in respect of whom the authorisation is made is employed in a palliative care service approved by the Secretary;(b) where the registered nurse is employed in a community health centre approved by the Secretary at which it is impractical for a medical practitioner to attend and the nurse is acting in accordance with the instructions of a medical practitioner;(c) where the registered nurse is employed in a medical institution approved by the Secretary at which it is impractical for medical practitioners to attend after hours and the nurse is acting in accordance with the instructions of a medical practitioner;(d) in the case of the possession or supply of lignocaine, where the registered nurse is employed by the Australian Red Cross Blood Service and is acting in the course of his or her professional practice;(e) where the registered nurse is employed in an in-vitro fertilisation clinic approved by the Secretary and the nurse is acting in accordance with the instructions of a medical practitioner;(f) where the Director of Public Health makes a declaration under section 14(1) of the Public Health Act 1997 that a public health emergency exists.
Division 6 - Miscellaneous
63. Prescribed form of various certificates, applications and licences
(1) For the purposes of section 25 of the Act –(a) a certificate of the result of an analysis under subsection (1) of that section is to be in accordance with Form 3; and(b) a certificate of the result of an examination under subsection (1A) of that section is to be in accordance with Form 4; and(c) a certificate of the results of an inspection under subsection (2) of that section is to be in accordance with Form 5.(2) For the purposes of section 27 of the Act –(a) an application for a licence to sell or supply substances to which that section applies is to be in accordance with Form 6 and contain particulars specified in the form; and(b) a licence in that behalf is to be in accordance with Form 7.(3) For the purposes of section 28(3) of the Act, a securely bound book containing pages in accordance with Form 8 and numbered consecutively is prescribed as the poisons book.
64. Prescribed persons for section 38(1)(i) of Act
For the purposes of section 38(1)(i) of the Act –(a) a person who is a dental therapist may, in the course of dental therapy, administer the following restricted substances to another person in the following specified circumstances:(i) demeclocycline (demethylchlortetracycline), when used in dental preparations;(ii) local anaesthetics, for use in dental treatment;(iii) topical fluorides, when used in dental preparations;(iv) triamcinolone acetonide, when used in dental preparations; and(b) a person who is a registered nurse may, in the course of nursing practice, administer to another person a substance listed in Schedule 2 or 3 to the Poisons List; and(c) a person who is a registered nurse and who –may administer to another person a vaccine listed in Schedule 4 to the Poisons List in accordance with a vaccination program approved by the Director of Public Health.(i) has completed an educational program approved by the Secretary relating to the administration of vaccines; and(ii) has been approved by the Secretary to administer vaccines independently –
65. Prescribed class 1 substances for section 38(1) of Act
The substances specified in Schedule 7 are prescribed as class 1 substances for the purposes of section 38(1) of the Act.
66. Restrictions on purchase, &c., of certain pesticidal substances
(1) A person must not –unless the person buying, obtaining, using or being supplied with the substance has the authority in writing of a competent officer to buy, obtain, use or be supplied with that substance.(a) buy or obtain or use –(i) fluoroacetic acid and substances structurally derived from fluoroacetic acid; or(ii) thallium; or(iii) thallium salts; or(b) sell or supply any substance referred to in paragraph (a) to any person –Penalty: Fine not exceeding 10 penalty units.(2) Every person who has a written authority referred to in subregulation (1) must comply with such conditions as are specified in the authority.Penalty: Fine not exceeding 10 penalty units.(3) A competent officer may, in his or her absolute discretion, suspend or cancel any authority given under subregulation (1) .(4) In this regulation –competent officer means –(a) the Registrar of Chemical Products; or(b) the Secretary of the Department of Primary Industries; or(c) an employee in the Department of Primary Industries authorised, in writing, by the Secretary of that Department to perform the functions of a competent officer under this regulation;Department of Primary Industries means the department that is responsible, in relation to the Vermin Control Act 2000 (or such other Act as from time to time has effect in substitution for that Act), to the Minister to whom the administration of that Act is assigned.
67. Veterinary use of chloramphenicol
(1) A person must not –(a) put chloramphenicol to a prohibited use; or(b) have possession of chloramphenicol for the purpose of its being put to a prohibited use; or(c) in any advertisement, in any label, leaflet or other document included in, attached to or otherwise accompanying a container containing chloramphenicol, or in any other way in connection with the sale or supply of chloramphenicol, represent chloramphenicol as being suitable to be put to a prohibited use.Penalty: Fine not exceeding 10 penalty units.(2) For the purposes of subregulation (1) , the following uses are prohibited uses:(a) systemic administration to a food-producing animal;(b) topical administration (other than ocular topical administration) to an animal.
68. Restriction on possession, &c., of diazepam
(1) A person who is not the holder of a licence under section 16(1) of the Act to carry on business as a manufacturing chemist or as a wholesale chemist must not have possession of a quantity of diazepam in an undivided state that –(a) where the person is the holder of a permit granted by the Secretary for the purposes of this regulation, exceeds the quantity specified in the permit; or(b) in any other case, exceeds 5 grams.Penalty: Fine not exceeding 10 penalty units.(2) For the purposes of subregulation (1) , diazepam is taken to be otherwise than in an undivided state only if it is in the form of tablets or capsules or it is packed in a selected container.(3) A permit granted for the purposes of this regulation is subject to any conditions specified in the permit.
69. Restriction on sale, &c., of clozapine
(1) Subject to subregulation (2) , a person must not prescribe, sell or supply clozapine unless the person is –(a) authorised by the Secretary to do so; or(b) included in a class of persons authorised by the Secretary to do so.Penalty: Fine not exceeding 10 penalty units.(2) Subregulation (1) does not apply to the sale or supply of clozapine by a wholesale chemist in the ordinary course of wholesale dealing.(3) A person authorised by this regulation to prescribe, sell or supply clozapine must comply with such conditions as are specified in the authority.Penalty: Fine not exceeding 10 penalty units.(4) The Secretary may at any time revoke an authority given under this regulation.
70. Prescription, &c., of certain substances prohibited, unless authorised by Secretary
(1) This regulation applies to the following substances:(a) acitretin;(b) bexarotene for human use;(c) bosentan for human use;(d) clomiphene;(e) cyclofenil;(f) dinoprost;(g) dinoprostone;(h) dronabinol (S8);(i) etretinate;(j) follitropin alpha (recombinant human follicle-stimulating hormone) for human use;(k) follitropin beta (recombinant human follicle-stimulating hormone) for human use;(l) urofollitrophin (otherwise known as follicle-stimulating hormone);(m) isotretinoin for human oral use;(n) luteinising hormone (S4);(o) sitaxentan;(p) thalidomide;(q) tretinoin for human oral use.(2) Subject to subregulation (3) , a person must not prescribe, sell or supply a substance to which this regulation applies for human use unless that person is –(a) authorised by the Secretary to do so; or(b) included in a class of persons authorised by the Secretary to do so.Penalty: Fine not exceeding 10 penalty units.(3) Subregulation (2) does not apply to the sale or supply of any substance specified in that subregulation –(a) by a wholesale chemist in the ordinary course of wholesale dealing; or(b) by a pharmaceutical chemist on the prescription of a person who has the authority of the Secretary to prescribe the substance.(4) A person authorised under this regulation to prescribe or supply any of the substances specified in this regulation must comply with such conditions as are specified in the authority.Penalty: Fine not exceeding 10 penalty units.(5) The Secretary may at any time revoke an authority given under this regulation.
71. Precautions in prescribing substances capable of producing birth defects
(1) A medical practitioner must not prescribe or supply for use in the treatment of a female patient acitretin, bexarotene, bosentan, etretinate, sitaxentan, isotretinoin for oral use, tretinoin for oral use or thalidomide unless –(a) the medical practitioner is satisfied that, at that time, the patient is not pregnant; and(b) the medical practitioner –(i) is satisfied that the patient is incapable, after that time, of becoming pregnant; or(ii) warns the patient that, if she is, or becomes, pregnant at any time during the period specified in subregulation (2) , any child of that pregnancy is extremely likely to suffer severe birth defects.Penalty: Fine not exceeding 10 penalty units.(2) The period referred to in subregulation (1)(b)(ii) is the period –(a) commencing at the commencement of the period of treatment of the patient with acitretin, bexarotene, bosentan, etretinate, sitaxentan, isotretinoin for oral use, tretinoin for oral use or thalidomide, as the case requires; and(b) ending –(i) in the case of treatment with acitretin or etretinate, 2 years after the completion of that treatment; or(ii) in the case of treatment with bosentan or sitaxentan, 3 months after the completion of that treatment; or(iii) in the case of treatment with isotretinoin for oral use, tretinoin for oral use, bexarotene or thalidomide, one month after the completion of that treatment.
72. Restrictions on use of certain poisons and restricted substances
(1) A person must not, without the approval in writing of the Secretary or of the Secretary of the Commonwealth Department –(a) prescribe, or sell or supply, a substance specified in a succeeding subregulation of this regulation for a use that is not permitted in relation to that substance under that subregulation; or(b) put such a substance to such a use; or(c) in any advertisement or in any label, leaflet or other document included in, attached to or otherwise accompanying a container containing such a substance, or in any other way in connection with the sale or supply of such a substance, represent that substance to be suitable for such a use.Penalty: Fine not exceeding 10 penalty units.(2) A person must not put to human therapeutic use a substance listed in Appendix C of the Uniform Standard.Penalty: Fine not exceeding 10 penalty units.(3) A person must not use S,S,S-tributylphosphorothioate (S7) as a pesticide.Penalty: Fine not exceeding 10 penalty units.(4) A person must not use nitrofuran –(a) in animal feed; or(b) for the treatment of an animal used for the production of meat, edible offal, egg or milk.Penalty: Fine not exceeding 10 penalty units.
73. Restriction of possession of certain substances
A person must not have possession of a substance specified in Appendix C of the Uniform Standard otherwise than in accordance with the approval in writing of either the Secretary or the Secretary of the Commonwealth Department.Penalty: Fine not exceeding 10 penalty units.
74. Restrictions on certain dangerous poisons
A person must not manufacture, obtain, possess, sell, supply or use a dangerous poison to which Condition 1 in Part 1 of Appendix J of the Uniform Standard applies except –(a) if the person –(i) is authorised, or is a member of a class of persons authorised, by the Secretary; and(ii) is acting in accordance with that authorisation; or(b) for any purposes and on any conditions –(i) set out in these regulations; or(ii) approved by the Secretary; or(c) in accordance with a licence in force under the Dangerous Goods Act 1998 or any other authorisation under any relevant Act.Penalty: Fine not exceeding 10 penalty units.
75. Restrictions applying to veterinary medicines
A person must not –a medicine or other substance which contains a poison or restricted substance and which is prepared for the treatment of animals.(a) administer to himself or herself; or(b) administer to another person; or(c) sell or supply for human use; or(d) represent as being suitable for human use –Penalty: Fine not exceeding 10 penalty units.
(1) The professions, businesses, trades or industries carried on by persons specified in subregulations (2) , (3) and (4) are prescribed for the purposes of the definition of "wholesale dealing" in section 3(1) of the Act.(2) In respect of narcotic substances (S8) the following are specified:(a) the holder of a licence to manufacture, use or possess narcotic substances in force under regulation 7 ;(b) the holder of a licence to sell, distribute and supply narcotic substances in force under Division 2 of Part V of the Act;(c) an authorised officer who is not a pharmaceutical chemist;(d) the master of a vessel, if the substances are intended to be used only for medical treatment on the vessel and are needed to complete the quantity of medicines and medical stores required to be carried on the vessel to comply with navigation requirements;(e) a registered nurse authorised in respect of those substances under section 25A of the Act;(f) a person approved to keep a first aid kit under regulation 92 ;(g) a person directed under regulation 38 by the Director of Ambulance Services to procure and distribute those substances for the Ambulance Service.(3) In respect of restricted substances (S4) and potent substances (S3) the following are specified:(a) a qualified person in charge of a laboratory or department engaged in medical or scientific research or instruction or in quality control or analysis;(b) the holder of a licence to make or refine those substances in force under Division 3 of Part II of the Act;(c) the holder of a licence to buy and sell those substances in force under Division 3 of Part II of the Act;(d) an authorised officer who is not a pharmaceutical chemist;(e) the master of a vessel, if those substances are intended to be used only for medical treatment on the vessel and are needed to complete the quantity of medicines and medical stores required to be carried on the vessel to comply with navigation requirements;(f) a registered nurse authorised in respect of those substances under section 25A of the Act;(g) a person approved to keep a first aid kit under regulation 92 ;(h) a person directed under regulation 38 by the Director of Ambulance Services to procure and distribute those substances for the Ambulance Service;(i) a registered optometrist within the meaning of the Optometrists Registration Act 1994 ;(j) in respect of the substances specified in regulation 64 , a registered nurse referred to in paragraph (c) of that regulation or a dental therapist.(4) In respect of other scheduled substances not specified in regulation 66 the following are specified:(a) a qualified person in charge of a laboratory or department engaged in medical or scientific research or instruction or in quality control or analysis;(b) the holder of a licence to make or refine those substances in force under Division 3 of Part II of the Act;(c) the holder of a licence to buy and sell those substances in force under Division 3 of Part II of the Act;(d) an authorised officer who is not a pharmaceutical chemist;(e) the master of a vessel, if those substances are intended to be used only for medical treatment on the vessel and are needed to complete the quantity of medicines and medical stores required to be carried on the vessel to comply with navigation requirements;(f) a person having control of an industrial first aid post;(g) a person directed under regulation 38 by the Director of Ambulance Services to procure and distribute those substances for the Ambulance Service;(h) a registered optometrist within the meaning of the Optometrists Registration Act 1994 ;(i) a person engaged in the occupation of jewellery manufacture;(j) a person engaged in the occupation of electroplating;(k) a person engaged in the occupation of paint manufacture;(l) a person engaged in the occupation of ferrous hardening;(m) a person engaged in commercial pest control.
(1) A person who sells or supplies a poison or restricted substance in the ordinary course of wholesale dealing must, on each occasion upon which the sale or supply is made –(a) issue an invoice to the purchaser of the substance or the person to whom the substance is supplied; and(b) keep a record of the invoice showing –(i) the date of the sale or supply; and(ii) the name and address of the purchaser or the person to whom the substance is supplied; and(iii) the name and quantity of the substance sold or supplied.Penalty: Fine not exceeding 10 penalty units.(2) A person who sells or supplies any poison or restricted substance must keep any invoice and prescription record relating to that poison or restricted substance for not less than 2 years from the latest date on which the invoice or prescription record was made or acted upon.Penalty: Fine not exceeding 10 penalty units.(3) On demand by a person appointed or authorised under section 23 of the Act, any person authorised to supply, sell or be in possession of any poison or restricted substance must furnish particulars of the quantity of any poison or restricted substance on hand, the quantity obtained and the quantity disposed of.Penalty: Fine not exceeding 10 penalty units.
78. Supply of clinical samples
(1) This regulation applies to –who lawfully supplies any of those substances by way of free distribution as a clinical sample.(a) a person engaged in the manufacture of, or wholesale dealing in, any substance specified in Schedule 1 or 4 to the Poisons List; and(b) the agent of any such person –(2) Any person to whom this regulation applies must, on each occasion upon which the person supplies any substance referred to in subregulation (1) by way of free distribution, make a record of the supply showing –(a) the date of the supply; and(b) the name and address of the person to whom the substance was supplied; and(c) the name and quantity of the substance so supplied.(3) Except in the case of the supply of the substance referred to in subregulation (1) by registered or certified mail, a person supplying the substance must obtain a receipt at the time of supply from the person to whom the supply was made.(4) The provisions of regulation 77(2) and (3) apply, with any necessary modification, to the records and receipts required to be made under this regulation.
PART 5 - Packaging and Labelling of Scheduled Substances
Division 1 - Application of provisions of Uniform Standard
79. Application of provisions of Uniform Standard
(1) Subject to subregulation (3) and to any provision to the contrary in these regulations, Part 2, paragraph 41 in Part 3 and Appendices E, F and J in Part 5 of the Uniform Standard (in this regulation referred to as "the applied provisions") have effect as if they were provisions of these regulations.(2) For the purposes of subregulation (1) –(a) a reference in any of the applied provisions to a Schedule by number is to be read as a reference to the correspondingly numbered Schedule to the Poisons List; and(b) an expression that is defined in Part 1 of the Uniform Standard has, unless the contrary intention appears, the corresponding meaning in the applied provisions; and(c) a reference in any of the applied provisions to "authorised or licensed persons" is to be read as a reference to a person authorised, licensed, permitted or approved under any relevant Act.(3) The Minister may, by permit signed by the Minister, in such circumstances as the Minister thinks fit, authorise the sale or supply of a scheduled substance the labelling or packaging of which does not comply with a requirement of the applied provisions.(4) A permit under subregulation (3) has effect subject to any conditions specified in the permit.(5) A person must comply with paragraph 2 in Part 2 of the Uniform Standard.Penalty: Fine not exceeding 10 penalty units.
Division 2 - Packaging of scheduled substances
80. Special provisions as to containers for narcotic substances
(1) A person must not sell or supply a narcotic substance unless its container is sealed in such a way that, when the seal is broken, the container can be readily distinguished from sealed containers.Penalty: Fine not exceeding 10 penalty units.(2) Where more than one container containing a narcotic substance is enclosed in a primary pack, a person must not sell or supply a narcotic substance in such a primary pack unless that primary pack is sealed in such a way that, when the seal is broken, the primary pack can be readily distinguished from sealed primary packs.Penalty: Fine not exceeding 10 penalty units.(3) This regulation does not apply to the sale or supply of a narcotic substance –(a) by a medical practitioner, dentist, authorised nurse practitioner or veterinary surgeon in the practice of his or her profession; or(b) by a pharmaceutical chemist on the prescription of a medical practitioner, dentist, authorised nurse practitioner or veterinary surgeon; or(c) by a registered nurse on the authorisation in writing of a medical practitioner, dentist or authorised nurse practitioner.
81. Child-resistant packaging of certain medicines
If goods to which the Therapeutic Goods Order No. 65, made under the Therapeutic Goods Act 1989 of the Commonwealth, as amended from time to time, applies consist of, or include, a scheduled substance, the provisions of that order, or any order made in substitution of that order, have effect for the purposes of the Act in relation to those goods as if those provisions were provisions of these regulations.
Division 3 - Labelling of scheduled substances
82. Labelling of poisons in poison book
A person must not sell any poison, the sale of which requires an entry to be made in the poisons book, unless the person so selling has first affixed to the container in which the poison is sold a label on which is written the seller's name and address which may appear on a label separate from the principal label.Penalty: Fine not exceeding 10 penalty units.
A person must not sell or supply –(a) a substance included in a Schedule to the Poisons List in a container to which is affixed a label on which is written –(i) a reference to the Act or to these regulations, or any comment upon, or any reference to or any explanation of, any information required by the Act or by a regulation to be written on any label, which directly or by implication contradicts, qualifies or modifies such information; or(ii) any device which is, or any words which are, false or misleading in any particular concerning the substance or any one or more of the ingredients included in it; or(b) any poison, restricted substance or narcotic substance in a container on which is affixed a label which obscures –(i) any expression required by the Uniform Standard as applied by regulation 79 to be written or embossed on that container; or(ii) any ribs, grooves, points or stars required by the Uniform Standard as applied by regulation 79 to be embossed on that container; or(iii) any words required by the Act or these regulations to be written on such a container or on a label affixed to that container.Penalty: Fine not exceeding 10 penalty units.
84. Labelling of dispensed medicines
(1) A scheduled substance when –is exempt from all other provisions of these regulations relating to labels if the container of the substance is labelled in accordance with subregulation (2) .(a) made up or compounded as a medicine by a pharmaceutical chemist acting in the lawful practice of his or her profession as such, or by an assistant under his or her direct personal supervision, on and in accordance with the prescription of a medical practitioner, dentist or veterinary surgeon or an authorised optometrist; or(b) made up or compounded extemporaneously as a medicine by a pharmaceutical chemist so acting for a specific and individual case, if the medicine does not contain any restricted substance or narcotic substance; or(c) made up or compounded as a medicine which is supplied –(i) by a medical practitioner so acting for the purposes of medical treatment; or(ii) by a dentist so acting for the purposes of dental treatment; or(iii) by a veterinary surgeon so acting for the purposes of animal treatment; or(iv) by an authorised optometrist so acting for the purposes of optometrical treatment –(2) For the purposes of subregulation (1) , the container is to be labelled with –(a) the words "Keep out of reach of children" in red on a white background; and(b) the name of the patient or in the case of an animal the name of the owner of the animal; and(c) the name and address of the seller; and(d) such additional inscriptions as are required by subregulation (3) to be included.(3) For the purposes of subregulation (2)(d) , the labelling of the container of the medicine is to include additional inscriptions as follows:(a) where the medicine is for external use – the word "Poison", or the words "Caution – Not to be taken" or "Do not swallow", in red;(b) where the medicine is, or includes, a substance in relation to which, but for this regulation, the container would be required, under the provisions of the Uniform Standard having effect under regulation 79 , to be labelled with a warning relating to pregnancy or to the effect that that substance causes, or may cause, birth defects – that warning, together with any inscription required by that regulation to be prefixed to that warning, in the form specified in that regulation;(c) where the medicine is for internal use and contains a substance specified in Appendix K to the Uniform Standard – a warning statement in the form, or to the effect, of warning statement 39 or warning statement 40 in Part 1 of Appendix F to the Uniform Standard;(d) where the medicine is levocabastine, a warning statement in the form, or to the effect, of warning statement 62 in Part 1 of Appendix F to the Uniform Standard;(e) where the medicine is acitretin, adapalene, bexarotene, bosentan, etretinate, isotretinoin, sitaxentan, thalidomide or tretinoin –(i) for oral use, warning statements in the form, or to the effect, of warning statements 7, 62 and 76 in Part 1 of Appendix F to the Uniform Standard; or(ii) for topical use, warning statements in the for, or to the effect, of warning statements 62 and 77 in Part 1 of Appendix F to the Uniform Standard;(f) where the medicine is leflunomide, warning statements in the form, or to the effect, of warning statements 7, 62 and 87 in Part 1 of Appendix F to the Uniform Standard;(g) where the medicine is misoprostol, a warning statement in the form, or to the effect, of warning statement 53 in Part 1 of Appendix F to the Uniform Standard;(h) where the medicine is dienestrol, a warning statement in the form, or to the effect of, warning statement 67 in Part 1 of Appendix F to the Uniform Standard;(i) where the medicine is for animal treatment – the words "For animal treatment only";(j) where the medicine is dispensed in accordance with a prescription –(i) the particulars set out in the prescription to be included on the label; and(ii) the name of the substance shown in the prescription unless –(A) otherwise directed in the prescription; or(B) the substance is a medicine compounded extemporaneously in accordance with the formula set forth in the prescription;(k) where the medicine is not dispensed in accordance with a prescription –(i) adequate directions for its use specifically in the treatment of the patient or animal for whose treatment it was supplied; and(ii) the name and strength of the scheduled substance.(4) A number or letter required on a label is to be –(a) at least 1·5 mm high; and(b) in clear and distinct contrast to the background.(5) The Secretary may approve a variation to the labelling requirements set out in this regulation subject to any condition he or she considers appropriate.
PART 6 - Miscellaneous
85. Revocation of licence to be published
If, under section 92(1) of the Act, the Minister suspends or revokes a right conferred on a person by or under the Act, the Minister, if of the opinion that it is in the public interest to do so, may do any or all of the following:(a) give notice of the suspension or revocation to any professional registration authority;(b) cause notice of the suspension or revocation to be published in any professional publication;(c) cause notice of the suspension or revocation to be published in a newspaper;(d) cause notice of the suspension or revocation to be published in the Gazette;(e) give notice of the suspension or revocation to any employer or other relevant person or body.
86. Offence to act if right suspended or revoked
A person must not –during any period in which a right conferred on the person to do so has been suspended or revoked by the Minister under section 92(1) of the Act.(a) make, refine, prepare, prescribe, sell, supply or have in his or her possession a scheduled substance, prohibited substance or raw narcotic; or(b) grow, cultivate, use or have in his or her possession a prohibited plant –Penalty: Fine not exceeding 10 penalty units.
87. Duration of licence or authority
(1) A licence or authority has effect until the date specified in it, unless it is renewed or cancelled.(2) Where an application for the renewal of a licence or authority is refused –(a) that licence or authority continues in force until the expiration of the period ordinarily limited for the bringing of an appeal against that refusal; and(b) if such an appeal is brought, the licence or authority continues until the final determination or abandonment of that appeal.(3) A licence issued to a person to carry on business as a manufacturing chemist ceases to have effect upon that person ceasing to be a qualified person.(4) A licence to grow or cultivate a prohibited plant issued under Part V of the Act in a certain year ceases to have effect on 31 December in the following year.
88. Surrender of licence or authority
(1) If a person holding a licence or authority surrenders it to the Secretary, the licence or authority ceases to have effect.(2) Where a licence or authority ceases to have effect otherwise than on its revocation, the person by whom the licence or authority is held must deliver that licence or authority to the Secretary.Penalty: Fine not exceeding 10 penalty units.
89. Application to extend suspension
(1) For the purposes of section 92(3) of the Act, an application is to –(a) be in writing; and(b) set out the grounds upon which the application is made; and(c) be served on, or forwarded by certified mail to, the magistrate before the period of suspension expires.(2) A copy of the application is to be served on, or forwarded by certified mail to, the person in respect of whom an application is being made under subregulation (1) .
For the purposes of section 92A(2)(c) of the Act, the following are prescribed authorities:(a) the National Health and Medical Research Council, constituted under the National Health and Medical Research Council Act 1992 of the Commonwealth;(b) the National Drugs and Poisons Schedule Committee, constituted under the Therapeutic Goods Act 1989 of the Commonwealth.
These regulations do not apply to –(a) a poison in a product listed in Appendix A to the Uniform Standard; or(b) a poison listed in column 1 of Appendix G to the Uniform Standard, at a concentration not exceeding the concentration specified in respect of the poison in column 2 of that Appendix; or(c) a poison listed in Schedules 1 to 6 to the Poisons List at a concentration not exceeding 10mg per litre or 10mg per kilogram unless that poison is also listed in Schedule 7 or 8 to the Poisons List; or(d) a plant listed in any of Schedules 1 to 7 , inclusive, to the Poisons List, or any part of that plant, except when packed or prepared for therapeutic use.
(1) The Secretary may, by instrument in writing, grant approval for a first aid kit to be kept in a place, locality or vehicle specified in the instrument for use only in the case of an emergency by –(a) a person in an isolated locality where workers are employed; or(b) a person representing an organisation established for search and rescue in a mountainous or isolated area; or(c) another person specified in the instrument.(2) Subject to these regulations and any conditions that may from time to time be imposed by the Secretary in any particular case, a person who is –may possess, as part of that first aid kit, any narcotic substance, restricted substance or potent substance approved by the Secretary for installation in an approved first aid kit for use, in an emergency, by a person referred to in section 48 of the Act or regulation 9 .(a) for the time being in control of an approved first aid kit; or(b) with the approval of the Secretary, designated by the person for the time being having control of an approved first aid kit as a first aid officer –(3) An approval granted by the Secretary under this regulation may be revoked by the Secretary at any time.
(1) Except as otherwise approved by the Secretary –(a) a pharmaceutical chemist or dispensing medical practitioner is to provide the Secretary within 3 days after the end of each month with a return, in a form approved by the Secretary, giving details of all acquisitions and disposals of relevant substances during that month; and(b) a licensed manufacturing chemist or licensed wholesale chemist is to provide the Secretary within 3 days after the end of each week with a return in duplicate, in a form approved by the Secretary, giving details of all acquisitions and disposals of relevant substances during that week.(2) If a person to whom subregulation (1) applies does not make any acquisitions or disposals of relevant substances during any period for which the person is required to provide the Secretary with a return, the person is to provide a "nil" return.(3) For the purposes of this regulation –(a) a week begins at midnight on Saturday and ends at midnight on the following Saturday; and(b) a "relevant substance" means a substance in relation to which the Secretary determines a return is to be provided.
94. Administration of drugs by midwives under general orders
(1) In this regulation –drug means any of the following substances:(a) ergometrine;(b) metoclopramide;(c) morphine;(d) oxytocin;(e) pethidine;(f) promethazine;general order means an order issued by a medical practitioner under subregulation (2) ;hospital means a hospital that provides obstetric services;midwife means a registered nurse who holds an authorisation to practise midwifery under the Nursing Act 1995 .(2) Subject to this regulation, a medical practitioner may issue an order to the Director of Nursing of a hospital authorising a midwife who practises midwifery in that hospital to, in the midwife's discretion, do any one or more of the following things in that hospital in accordance with the order:(a) administer any drug specified in the order to a patient of that medical practitioner;(b) administer naloxone neonatally to a child born of a patient of that medical practitioner;(c) administer hepatitis B vaccine neonatally to a child born of a patient of that medical practitioner.(3) A medical practitioner must not issue a general order that purports to authorise a midwife to –(a) administer a scheduled substance other than a drug, naloxone or hepatitis B vaccine; or(b) administer a drug otherwise than in accordance with subregulation (9) ; or(c) administer naloxone otherwise than in accordance with subregulation (10) .(4) A general order is to –(a) be in writing; and(b) specify which substances may be administered under the order; and(c) state the name of the hospital in respect of which it is issued; and(d) be signed and dated by the medical practitioner issuing the order.(5) A general order is taken to have been issued when it is received by the Director of Nursing of the hospital to which it applies.(6) A medical practitioner must not vary a general order once it has been issued.(7) A medical practitioner may revoke a general order at any time by giving not less than 24 hours' written notice of the revocation to the Director of Nursing of the hospital in respect of which the order was issued.(8) If a medical practitioner issues a general order in respect of a hospital, a midwife who practises midwifery in that hospital may, in his or her discretion, do any one or more of the following things in that hospital in accordance with the order:(a) administer drugs to a patient of that medical practitioner;(b) administer naloxone neonatally to a child born of a patient of that medical practitioner;(c) administer hepatitis B vaccine neonatally to a child born of a patient of that medical practitioner.(9) For the purposes of this regulation, a general order may authorise the administration of drugs to a patient in the following doses and quantities and in the following manner of administration:(a) in the case of ergometrine – one dose not exceeding 0.5mg administered intramuscularly after delivery of the patient's child;(b) in the case of metoclopramide – one dose not exceeding 10mg administered intramuscularly;(c) in the case of morphine – one dose not exceeding 5mg administered intramuscularly;(d) in the case of oxytocin – one dose not exceeding 10 international units administered intravenously or intramuscularly after delivery of the patient's child;(e) in the case of pethidine – one dose not exceeding 50mg administered intravenously or one dose not exceeding 100mg administered intramuscularly;(f) in the case of promethazine – 2 doses, neither exceeding 25mg, administered intramuscularly.(10) A general order may authorise one dose of naloxone, not exceeding 0.02mg, to be administered neonatally.(11) A midwife who administers a drug, naloxone or hepatitis B vaccine to a person in accordance with a general order must enter details of the administration in that person's drug therapy record.
95. Administration of Schedules 2 , 3 and 4 substances
A person may administer to another person a substance listed in Schedule 2 , 3 or 4 to the Poisons List if –(a) the person administering the substance is –(i) employed by a disability services program approved by the Secretary or employed by a disability service provider who is funded by, and is the subject of a service agreement with, the Department; and(ii) acting –(A) in accordance with the Guidelines for the Administration of Medication for People with Disabilities in Community Based Disability Services, as issued by the Secretary and amended from time to time; or(B) in the case of a health professional, in the course of his or her professional practice; and(b) the person to whom the substance is administered –(i) is receiving services from a disability services program approved by the Secretary or from a disability service provider who is funded by, and is the subject of a service agreement with, the Department; and(ii) is incapable of safely administering the substance to himself or herself; and(c) in the case of a substance listed in Schedule 3 to the Poisons List, the substance has been supplied by a pharmaceutical chemist, medical practitioner, dentist or authorised nurse practitioner for the use of the person to whom it is administered; and(d) in the case of a substance listed in Schedule 4 to the Poisons List, the substance has been prescribed or supplied by a medical practitioner, dentist or authorised nurse practitioner for the use of the person to whom it is administered.
96. Administration of certain substances to detainees
A person may administer to a detainee a substance specified in Schedule 2 , 3 , 4 or 8 to the Poisons List if –(a) the person administering the substance –(i) is an employee employed at a detention centre; and(ii) is acting –(A) in accordance with guidelines approved by the Secretary; or(B) in the case of a health professional, in the course of his or her professional practice; and(b) the detainee is incapable of safely administering the substance to himself or herself; and(c) in the case of a substance listed in Schedule 3 to the Poisons List, the substance has been supplied by a pharmaceutical chemist, medical practitioner, dentist or authorised nurse practitioner for the use of the detainee; and(d) in the case of a substance listed in Schedule 4 or 8 to the Poisons List, the substance has been prescribed or supplied by a medical practitioner, dentist or authorised nurse practitioner for the use of the detainee.
97. Possession of pseudoephedrine
(1) In this regulation –registered goods has the same meaning as in the Therapeutic Goods Act 1989 of the Commonwealth;therapeutic goods register means the Australian Register of Therapeutic Goods maintained under the Therapeutic Goods Act 1989 of the Commonwealth.(2) A person must not possess more than 240 milligrams of pseudoephedrine which is not in a form included in that part of the therapeutic goods register relating to registered goods, or must not possess more than 6 grams of pseudoephedrine in a form which is included in that part of the register, unless –(a) the person is a medical practitioner, pharmaceutical chemist, authorised nurse practitioner, registered nurse or veterinary surgeon who possesses the pseudoephedrine in the course of his or her professional practice; or(b) the person is licensed or authorised under the Act or these regulations to possess pseudoephedrine and is acting in accordance with that licence or authorisation; or(c) the person is the master of a vessel and the pseudoephedrine is intended to be used only for medical treatment on the vessel and is needed to complete the quantity of medicines and medical stores required or permitted to be carried on the vessel to comply with the law of the Commonwealth; or(d) the person is authorised in writing by the Secretary and is acting in accordance with that authorisation; or(e) the pseudoephedrine is prepared by a pharmaceutical chemist, medical practitioner or veterinary surgeon in the course of his or her professional practice; or(f) the pseudoephedrine is supplied to the person by a medical practitioner, authorised nurse practitioner or a veterinary surgeon in the course of his or her professional practice; or(g) the pseudoephedrine is supplied to the person by a pharmaceutical chemist on the written order of a medical practitioner, authorised nurse practitioner or veterinary surgeon.Penalty: Fine not exceeding 10 penalty units.
SCHEDULE 1 - Forms
Form 1
Regulation 5(2)(a) and 5(3)(a)
Form 2
Regulation 5(2)(b) and 5(3)(b)
Form 3
Form 4
Form 5
Form 6
Form 7
Form 8
SCHEDULE 2 - Requirements in relation to Narcotic Substances Register
PART 1 - Form of Narcotic Substances Register
Form
PART 2 - Rules for keeping Narcotic Substances Register
1. Interpretation(1) In this Schedule –authorised dispenser, in relation to a medical institution, means a person authorised by the authorised officer to dispense narcotic substances within that institution;narcotic substance, when used in relation to a register, means the narcotic substance to which that register relates;register means a narcotic substances register.(2) In respect of a register, a reference in this Schedule to a space by a letter or to a column by a number is to be construed as a reference to the space so lettered or to the column so numbered, as the case may be, in the form of the register contained in Part 1 of this Schedule.(3) For the purpose of this Schedule, in respect of a register –(a) a narcotic substance is taken to be in the possession of a person if it is in the possession of another person acting as the person's servant and under the person's orders; and(b) a narcotic substance that is delivered to an authorised dispenser, or to some person on the authorised dispenser's behalf, for use in the medical institution of which he or she is the authorised dispenser or to the dispensary of that medical institution is taken to be delivered into his or her possession and to remain in his or her possession until it is disposed of; and(c) a narcotic substance that is in a medical institution is taken, unless it is in the possession of the authorised dispenser at that institution, to be in the possession of the person in charge of that institution; and(d) a narcotic substance is taken to have been acquired where it is delivered, received or otherwise comes into the possession of a person required to keep the register or is manufactured by the person or by some person acting as that person's servant and under that person's orders; and(e) a narcotic substance is taken to have been disposed of if, being in the possession of the person required to keep the register –(i) it is supplied to some person other than a person acting as the person's servant and under the person's orders; or(ii) it is administered to any person; or(iii) it is destroyed or is converted or made up into another substance, whether or not that substance is a narcotic substance.(4) Without prejudice to the operation of subclause (3) , where a narcotic substance that is in the possession of an authorised dispenser is supplied for the purpose of being kept or used elsewhere than in the dispensary at the medical institution of which he or she is the authorised dispenser, that narcotic substance is taken, for the purposes of the register required to be kept by that authorised dispenser, to be disposed of.(5) Without prejudice to the operation of subclause (3) , where a narcotic substance is transferred from premises in respect of which a register is kept by a person to other premises in respect of which a register is kept by the person, that narcotic substance is taken, for the purposes of the register, to be acquired.
2. Particulars to be inserted in registers(1) Subject to this clause, where a register is required to be kept in respect of any narcotic substance there is to be entered –(a) in space (a), the name and address of the premises to which the register relates; and(b) in space (b), the name of the narcotic substance; and(c) in space (c), the code number supplied by the Secretary that represents the narcotic substance; and(d) in column (1), the date of the occurrence of the happening to which the entry relates; and(e) in column (2), the quantity of the narcotic substance acquired; and(f) in column (3), the quantity of the narcotic substance disposed of; and(g) in column (4), the quantity of the narcotic substance left on hand after any acquisition or disposal has taken place; and(h) in column (5), a code letter taken from Part 3 of this Schedule which represents the movements of the narcotic substance; and(i) in column (6), opposite every entry in the register, the initials of the person acquiring or disposing of the narcotic substance to which the entry relates; and(j) in column (7) –(i) particulars of every movement of the narcotic substance which was shown in column (5) by the code letter "F" or the code letter "X"; and(ii) in the case of a register kept by a medical practitioner, dentist, authorised nurse practitioner or authorised nurse, the name of any person supplied with a narcotic substance by that medical practitioner, dentist, authorised nurse practitioner or authorised nurse; and(iii) in the case of a register kept by a veterinary surgeon, a sufficient description of any animal for or in respect of which the veterinary surgeon has supplied a narcotic substance together with the name of the owner of the animal.(2) A licensed wholesale chemist or licensed manufacturing chemist is, instead of entering in a register the particulars required by subclause (1)(c) , (h) and (j) in relation to a narcotic substance, to enter in the register –(a) in space (c), the code name supplied by the Secretary that represents the narcotic substance; and(b) in column (5), a code supplied by the Secretary that represents the movement of the narcotic substance; and(c) in column (7), a code supplied by the Secretary, that represents the recipient of the narcotic substance.
3. Provisions relating to sheets of registerWhere a register comprises 2 or more sheets, those sheets are to be kept securely attached together and numbered serially.
PART 3 - Movement Codes to be used in Narcotic Substances Register
Code | Meaning | D | Dispensed or supplied on written order from medical practitioner. | R | Received. | C | Returned to supplier. | F | Formulated (indicate in remarks column of register what narcotic substance was obtained from the formulation and whether it was dispensed). | X | Lost, stolen, destroyed under supervision, taken by inspector or sold to another pharmaceutical chemist. (Give details in remarks column of register). |
SCHEDULE 3 - Requirements in relation to Ward Narcotic Substances Register
PART 1 - Form of Ward Narcotic Substances Register
Form
PART 2 - Rules for keeping Ward Narcotic Substances Register
1. Interpretation(1) In this Schedule –narcotic substance, when used in relation to a register, means the narcotic substance to which that register relates;register means a ward narcotic substances register.(2) In respect of a register, a reference in this Schedule to a space by a letter or to a column by a number is a reference to the space so lettered or to the column so numbered, as the case may be, in the form of the register contained in Part 1 of this Schedule.(3) For the purposes of this Schedule, in respect of a register –(a) a narcotic substance is taken to be in the possession of a person if it is in the possession of another person acting as the person's servant and under the person's orders; and(b) a narcotic substance that is in a medical institution is taken, unless it is in the possession of the authorised dispenser at that institution, to be in the possession of the person in charge of that institution; and(c) a narcotic substance is taken to have been acquired where it is delivered, received or otherwise comes into the possession of the person required to keep the register; and(d) a narcotic substance is taken to have been disposed of if, being in the possession of the person required to keep the register –(i) it is supplied to some person other than a person acting as the person's servant and under the person's orders; or(ii) it is administered to any person; or(iii) it is destroyed or is converted or made up into another substance, whether or not that substance is a narcotic substance.(4) Without prejudice to the operation of subclause (3) , where a narcotic substance that is in the possession of an authorised dispenser is supplied for the purpose of being kept or used elsewhere than in the dispensary at the medical institution of which he or she is the authorised dispenser, that narcotic substance is taken, for the purposes of the register required to be kept by the authorised dispenser, to be disposed of.(5) Without prejudice to the operation of subclause (3) , where a narcotic substance is transferred from the ward in respect of which a register is kept to another ward in respect of which another register is kept, that narcotic substance is taken, for the purposes of the register first mentioned, to be disposed of, and for the purposes of the register second mentioned, to be acquired.
2. Provisions relating to names and addressesWhere a register is required to be kept under regulation 29 , there are to be inserted in space (a) the name of the medical institution to which the register relates, the address of the institution, and a sufficient description of the ward or other room containing the enclosure to which the register relates.
3. Name of narcotic substanceThere is to be inserted in space (b) the name of the narcotic substance to which the register relates.
4. Particulars to be inserted when narcotic substance acquiredWhere any narcotic substance is acquired, the following are to be inserted in the register:(a) in column (1), the date on which it was acquired;(b) in column (2), a sufficient indication of the means by which it was acquired, whether by way of purchase, dispensing or otherwise;(c) in column (3), the name of the person, or a sufficient indication of the source, from whom or from which it was acquired;(d) in column (4), the amount acquired;(e) in column (5), the signature of the person making the entry or on whose instructions it is made.
5. Particulars to be inserted when narcotic substance disposed of, &c.(1) Where any narcotic substance is disposed of, the following are to be inserted in the register:(a) in column (6), the date on which it was disposed of;(b) in column (7), the amount disposed of;(c) in column (8) –(i) if it was administered to a person, a sufficient indication of the means by which it was administered; or(ii) if it was destroyed or lost, a sufficient indication that it was destroyed or lost;(d) where the drug is supplied for administration to a person, in column (9), the name of that person;(e) in column (11), the signature of the person by whom the drug was disposed of.(2) Where a narcotic substance is administered to a person and the nature or the amount of the narcotic substance so administered is checked by a person other than the person by whom it was administered, that other person is to insert his or her initials in column (10) against the entry relating to the administration of the narcotic substance.(3) Where a narcotic substance is disposed of –there is to be inserted in the entry relating to the disposal of the narcotic substance in column (12) the name in block capitals of the medical practitioner or the person and, in column (13), his or her signature.(a) on the instructions of a medical practitioner (not being instructions contained in a prescription issued by that medical practitioner); or(b) on the instructions of the person who has the possession of the narcotic substance or of a person acting on that person's behalf –(4) Where any narcotic substance is acquired or disposed of, there is to be inserted in column (14) the amount of that narcotic substance held immediately after that acquisition or disposal.
6. Provisions relating to sheets of registerWhere a register comprises 2 or more sheets, those sheets are to be kept securely attached together and numbered serially.
SCHEDULE 4 - Specified psychotropic substances
1. Alprazolam.
2. Anabolic and androgenic steroidal agents (S4).
3. Androisoxazole.
4. Benactyzine, and other substances structurally derived from diphenylmethane with ataractic properties.
5. Benzodiazepine derivatives not elsewhere specified in this Schedule (except clonazepam and midazolam).
6. Boldenone (otherwise known as dehydrotestosterone).
7. Bromazepam.
8. Bromides (S4).
9. Bromvaletone.
10. Butylchloral hydrate.
11. Captodiame.
12. Carbromal.
13. Cathine.
14. Chloral hydrate (S4).
15. Chlorbutol (S4).
16. Chlordiazepoxide.
17. Chlormethiazole.
18. Chlormezanone.
19. Clobazam.
20. Clonazepam.
21. Clorazepate.
22. Clostebol (otherwise known as 4-chlorotestosterone).
23. Codeine (S4).
24. Darbepoetin alfa.
25. Dextropropoxyphene.
26. Diazepam.
27. Diethylpropion.
28. Drostanolone.
29. Ephedrine.
30. Epoetin alfa.
31. Epoetin beta.
32. Erythropoietin.
33. Erythropoietins not elsewhere specified in this Schedule.
34. Ethchlorvynol.
35. Ethinamate.
36. Ethyloestrenol.
37. Fluoxymesterone.
38. Flurazepam.
39. Glutethimide.
40. Lorazepam.
41. Medazepam.
42. Meprobamate.
43. Mestanolone.
44. Mesterolone (otherwise known as methyldihydrotestosterone).
45. Methandienone (otherwise known as methandrostenolone).
46. Methandriol.
47. Methenolone.
48. Methylpentynol and other substituted alkynes for internal use.
49. Methyltestosterone.
50. Methyprylone.
51. Mibolerone.
52. Midazolam.
53. Nalbuphine.
54. Nandrolone (otherwise known as nortestosterone).
55. Nitrazepam.
56. Norethandrolone.
57. Oxandrolone.
58. Oxazepam.
59. Oxymesterone (otherwise known as hydroxymethyltestosterone).
60. Oxymetholone.
61. Paraldehyde.
62. Phentermine.
63. Prasterone.
64. Prazepam.
65. Pseudoephedrine (S4).
66. Somatotropin (human growth hormone).
67. Stanolone (otherwise known as dihydrotestosterone).
68. Stanozolol.
69. Temazepam.
70. Testosterone (S4).
71. Trenbolone (S4).
72. Triazolam.
73. Triclofos.
SCHEDULE 5 - Substances not to be supplied without approved information sheet
1. Acitretin.
2. Bexarotene for human use.
3. Bosentan for human use.
4. Dextropropoxyphene.
5. Etretinate.
6. Isotretinoin.
7. Sitaxentan.
8. Thalidomide.
SCHEDULE 6 - Specified potent substances
1. Acepifylline (S3).
2. Aminophylline (S3).
3. Dihydrocodeine (S3) in undivided preparations.
4. Salbutamol (S3).
5. Terbutaline (S3).
6. Theophylline (S3).
SCHEDULE 7 - Class 1 substances
1. Aciclovir.
2. Apraclonidine.
3. Atropine.
4. Bacitracin.
5. Betaxalol.
6. Bimatoprost.
7. Brimonidine.
8. Brinzolamide.
9. Carbachol.
10. Chloramphenicol.
11. Ciprafloxacin.
12. Dexamethasone.
13. Diclofenac.
14. Dipivefrin.
15. Dorzolamide.
16. Fluorometholone.
17. Flurbiprofen.
18. Framycetin.
19. Gentamicin sulfate.
20. Hydrocortisone.
21. Indomethacin.
22. Ketorolac trometamol.
23. Latanoprost.
24. Levobunolol.
25. Levocabastine (S2).
26. Lodoxamide (S2).
27. Neomycin.
28. Ofloxacin.
29. Olopatadine.
30. Phenylephrine (S2).
31. Pilocarpine.
32. Polymyxin.
33. Prednisolone.
34. Sodium cromoglycate (S2).
35. Tetracycline.
36. Timolol.
37. Tobramycin.
38. Travoprost.
39. Vidarabin.
SCHEDULE 8 - Procedures to be followed in a medical institution in case of loss, spillage, breakage or unintentional destruction of narcotic substances
1. Procedure if narcotic substance lostThe procedure to be followed in a medical institution if a narcotic substance is lost is as follows:(a) the health professional who discovers the loss must, as soon as practicable, inform another health professional;(b) both of those health professionals must, as soon as practicable, enter details of the loss in the narcotic substances register and sign that entry;(c) the health professional who discovered the loss must, as soon as practicable, report the loss to the authorised officer.
2. Procedure if narcotic substance spilt, broken, &c.The procedure to be followed in a medical institution if a narcotic substance is spilt, broken or unintentionally destroyed is as follows:(a) the person handling the narcotic substance when the spillage, breakage or destruction occurs must, as soon as practicable, inform a health professional;(b) the health professional and that person must both, as soon as practicable, enter details of the spillage, breakage or destruction in the narcotic substances register and sign that entry;(c) the health professional must, as soon as practicable, arrange for and witness the disposal of any residue of the narcotic substance in the presence of another health professional;(d) the health professional must, as soon as practicable, report the spillage, breakage or destruction to the authorised officer.
SCHEDULE 9 - Fees
Fee units | 1. | Licence under regulation 5 to manufacture and sell or supply – | (a) scheduled substances including narcotic substances | 450 | (b) narcotic substances | 350 | (c) scheduled substances other than narcotic substances | 115 | 2. | Licence under regulation 5 to sell or supply by wholesale dealing – | (a) scheduled substances including narcotic substances | 115 | (b) narcotic substances | 60 | (c) scheduled substances other than narcotic substances | 60 | 3. | Permit under regulation 6 to purchase a substance specified in Schedule 1 , 2 , 3 or 4 to the Poisons List for industrial, educational, advisory or research purposes | 25 | 4. | Copy of certificate showing the result of an analysis or examination issued under section 66 of the Act | 12 | 5. | Additional copy of certificate of analysis or certificate of inspection | 6 | 6. | Analysis carried out under section 63 of the Act – | (a) organic drugs – | (i) qualitative analysis | 45 | (ii) quantitative analysis | 60 | (b) inorganic drugs – | (i) qualitative analysis | 45 | (ii) quantitative analysis | 60 | (c) pesticides, herbicides, rodenticides, &c. – | (i) qualitative analysis | 45 | (ii) quantitative analysis | 60 | (d) poisons, not elsewhere included – | (i) organic – qualitative analysis | 45 | (ii) organic – quantitative analysis | 60 | (iii) inorganic – qualitative analysis | 45 | (iv) inorganic – quantitative analysis | 60 | 7. | Licence to sell or supply substances to which section 27 of the Act applies | 25 |
Displayed and numbered in accordance with the Rules Publication Act 1953.
Notified in the Gazette on 17 December 2008
These regulations are administered in the Department of Health and Human Services.
EXPLANATORY NOTE
(This note is not part of the regulation)
These regulations make provision in respect of the prescription, possession, supply, manufacturing, storage, labelling, packaging and disposal of narcotic substances, poisons and restricted substances and miscellaneous related matters.