Agricultural and Veterinary Chemicals (Control of Use) Regulations 2022
I, the Governor in and over the State of Tasmania and its Dependencies in the Commonwealth of Australia, acting with the advice of the Executive Council, make the following regulations under the Agricultural and Veterinary Chemicals (Control of Use) Act 1995 .
12 April 2022B. BAKER
Governor
By Her Excellency's Command,
JOANNE PALMER
Minister for Primary Industries and Water
PART 1 - Preliminary
These regulations may be cited as the Agricultural and Veterinary Chemicals (Control of Use) Regulations 2022 .
These regulations take effect on the day on which their making is notified in the Gazette.
(1) In these regulations, unless the contrary intention appears –Act means the Agricultural and Veterinary Chemicals (Control of Use) Act 1995 ;Agvet Regulations of Tasmania has the same meaning as in the Agricultural and Veterinary Chemicals (Tasmania) Act 1994 ;Animal Feedstuffs Residue Standard means –(a) the standard specified in table 4 of Part 2 of Schedule 1 to the Agricultural and Veterinary Chemicals Code (MRL Standard) Instrument 2019 of the Commonwealth; or(b) any document published by a Department of the Commonwealth Government to replace that table;authorised order form means an order form provided by the Registrar of Animal Brands under regulation 31 ;certificate of competency means a certificate of competency granted under regulation 11 ;chemical operator means a person engaged in handling a chemical product;current Poisons Standard has the same meaning as in the Agvet Regulations of Tasmania;Department of the Commonwealth Government includes an incorporated or unincorporated body which is established, constituted or continued by or under an Act of the Commonwealth or under the royal prerogative applied in respect of the Commonwealth of Australia, being a body which, or of which the governing authority, wholly or partly comprises a person or persons appointed by the Governor-General, a Minister of the Commonwealth Government or another similar body;HGP-Free Declaration means a declaration, contained in a national vendor declaration, that stock has not been treated with a hormonal growth promotant;HGP-Free tag means a tag registered under the Animal (Brands and Movement) Act 1984 for use to identify HGP-free stock;hormonal growth promotant purchaser declaration means a declaration made in accordance with regulation 48 of the Agvet Regulations of Tasmania;listed chemical product means a chemical product, or a chemical product of a class of chemical products, specified in Schedule 1 ;national vendor declaration means –(a) a declaration and waybill approved and published by SAFEMEAT as part of the Livestock Production Assurance system, as amended or substituted from time to time; or(b) in the case of pigs, a declaration (known as PigPass) approved and published by Australian Pork Limited (ABN 83 092 783 278) as amended or substituted from time to time.;Registrar of Animal Brands means the Registrar of Animal Brands appointed and holding office under section 5 of the Animal (Brands and Movement) Act 1984 ;SAFEMEAT means the consultative partnership, consisting of representatives from the Commonwealth government and the red meat and livestock industry, established to ensure the safety and hygiene of red meat and livestock;tail tag means a registered tail tag, within the meaning of the Animal (Brands and Movement) Act 1984 , which contains an identification of the property on which animals are managed by the proprietor of the tag.(2) In these regulations, unless the contrary intention appears, a reference to a form identified with a number is a reference to the form with that number contained in Schedule 2 .
(1) The fees set out in Schedule 3 are prescribed as the fees payable in respect of the matters to which they relate.(2) For the purposes of section 64 of the Act, the rate at which a fee due bears interest is 7% per annum.(3) For the purposes of the Act, an annual fee in respect of an authority, within the meaning of Schedule 5 of the Act, is due on the yearly anniversary of the authority.
PART 2 - Chemical Products Generally
5. Storage of chemical product
A person who keeps or has control of a chemical product must ensure –(a) that it is not readily accessible to children when it is being kept; and(b) that it will not, either as it is kept or in the event of the container in which it is kept breaking, leaking or being damaged, contaminate –(i) food intended for human consumption; or(ii) produce intended to be fed to stock; or(iii) goods or materials kept for sale.Penalty: Fine not exceeding 50 penalty units.
6. Prohibition on applying agricultural chemical product by irrigation
A person must not apply an agricultural chemical product by means of equipment designed for irrigation purposes unless –(a) the advice notice or label accompanying the agricultural chemical product specifies that it may be applied by those means; or(b) the person has a permit under section 29 of the Act which permits the application of the agricultural chemical product by those means.Penalty: Fine not exceeding 50 penalty units.
(1) If the Registrar has reason to believe that the health of a chemical operator may have been affected by the handling of a chemical product, the Registrar may require the chemical operator to undergo a medical examination –(a) by a registered medical practitioner at the time and place specified in the requirement; or(b) by a registered medical practitioner as arranged by the chemical operator and approved by the Registrar.(2) A requirement under subregulation (1) for a chemical operator to undergo a medical examination is to be made by written notice provided to the chemical operator.(3) A chemical operator must comply with a requirement to undergo a medical examination made under subregulation (2) .Penalty: Fine not exceeding 20 penalty units.(4) The employer of a chemical operator who is required under subregulation (1) to undergo a medical examination is to –(a) allow the chemical operator to be absent from employment for the purpose of attending the medical examination; and(b) if the chemical operator is absent from employment under paragraph (a) , pay the chemical operator the remuneration that the chemical operator would have received if he or she had remained at the place of employment during the time when he or she is absent for the purpose of attending the medical examination.(5) A person is to, on the demand of a chemical operator who is required under subregulation (1) to undergo a medical examination, reimburse the chemical operator for the reasonable expenses incurred by the chemical operator in complying with that requirement if –(a) the person employed the chemical operator in handling a chemical product or class of chemical products before the requirement was made; and(b) the requirement was made in relation to that chemical product or class of chemical products; and(c) the Registrar certifies that there are reasonable grounds for believing that the handling of that chemical product or class of chemical products on behalf of that person contributed to the need for making that requirement.(6) The Registrar may make any arrangements he or she considers appropriate to facilitate the carrying out of a medical examination required under subregulation (1) .(7) The medical practitioner carrying out a medical examination in response to a requirement made under subregulation (1) –(a) may take, or require the taking of, samples of blood and other substances; and(b) may have those samples tested; and(c) is to provide the Registrar with a report on the results of the medical examination and the testing of any samples taken.(8) If the Registrar is satisfied, after considering the report of the medical practitioner, that a chemical operator is absorbing or has absorbed a chemical product, or one or more chemical products of a class of chemical products, in quantities that are adversely affecting, or are likely to adversely affect, the health of the chemical operator, the Registrar –(a) must provide the chemical operator with a written notice stating that fact; and(b) may, by a further written notice provided to the chemical operator, prohibit the chemical operator from handling that chemical product or any chemical product of that class, or from performing the operations specified in the notice, indefinitely or for the period specified in the notice; and(c) must provide a copy of all notices provided under paragraphs (a) and (b) to all persons who appear to the Registrar to have employed the chemical operator to handle that chemical product or any chemical product of that class when, or after the time at which, the chemical operator was provided with the requirement to undergo a medical examination.(9) If a chemical operator has been prohibited, by a notice under subregulation (8)(b) , from handling a chemical product or any chemical product of a class of chemical products or from performing an operation –(a) the chemical operator must not, except with the written consent of the Registrar, contravene that notice; and(b) a person who has been provided with a copy of that notice must not, except with the written consent of the Registrar, employ the chemical operator to handle a chemical product or perform an operation in contravention of that notice.Penalty: Fine not exceeding 20 penalty units.
8. Chemical products requiring commercial chemical operator licence
(1) In this regulation –authorised person means a person who is –(a) appointed or employed under the State Service Act 2000 for the purposes of the Department; and(b) authorised by the Chief Veterinary Officer or the Registrar to apply the relevant agricultural chemical product, relevant veterinary chemical product or a class of chemical products of which the relevant agricultural chemical product or relevant veterinary chemical product is a member;small amount means the amount of a chemical product that the Registrar, by notice published –determines to be a small amount.(a) in the Gazette; or(b) on a website maintained by or on behalf of the Department; or(c) both –(2) The following classes of chemical products are prescribed for the purposes of section 21 of the Act:(a) any agricultural chemical product except when –(i) only a small amount of the agricultural chemical product is being used and that small amount is being applied by hand-held equipment for a purpose only incidental to the main service that is being provided for fee or other reward; or(ii) that agricultural chemical product is being applied by the Registrar, an authorised person or a veterinary surgeon in the course of his or her profession;(b) any veterinary chemical product except when –(i) only a small amount of the veterinary chemical product is being used and that small amount is being applied by hand-held equipment for a purpose only incidental to the main service that is being provided for fee or other reward; or(ii) that veterinary chemical product is being applied by the Registrar, an authorised person or a veterinary surgeon in the course of his or her profession.
9. Notification to neighbours of agricultural spraying
(1) The following classes of agricultural chemical products are prescribed for the purposes of sections 31(1)(a) and (2)(a) of the Act:(a) any agricultural chemical product listed, or which contains a constituent listed, in Schedule 6 to the current Poisons Standard;(b) any agricultural chemical product listed, or which contains a constituent listed, in Schedule 7 to the current Poisons Standard.(2) The following distances are prescribed for the purposes of sections 31(1)(b) and (2)(b) of the Act:(a) one kilometre if the agricultural chemical product is to be applied by aircraft;(b) 100 metres if the agricultural chemical product is to be applied by any other means.(3) The following manners of carrying out agricultural spraying are prescribed for the purposes of section 31(1)(c) of the Act:(a) spraying a liquid;(b) generating a mist;(c) dispersing a dust.
10. Application for certificate of competency to handle or use listed chemical products
(1) A person may apply to the Registrar for a certificate of competency in respect of that chemical product or the class of chemical products of which that chemical product is a member.(2) An application must –(a) be in a form approved by the Registrar; and(b) include any information that the Registrar reasonably requires; and(c) be lodged with the Registrar.
11. Grant or refusal of certificate of competency
(1) The Registrar may grant or refuse to grant a certificate of competency in respect of –(a) a listed chemical product; or(b) a class of chemical products of which a listed chemical product is a member.(2) The following are the types of certificate of competency which may be granted:(a) category 1 chemical user;(b) category 2 chemical user;(c) category 3 chemical user;(d) pest management technician;(e) methyl bromide or phosphine user.(3) A certificate of competency is subject to any conditions specified in it.(4) On determining to grant or refuse to grant a certificate of competency, the Registrar may, in writing –(a) require the applicant for the certificate to pay the prescribed application fee; and(b) require that fee to be paid within the time specified in the requirement.(5) A prescribed application fee required to be paid under subregulation (4)(a) is a debt due to the Crown and may be recovered in a court of competent jurisdiction.(6) The Registrar may do one or more of the following by notice in writing to the holder of a certificate of competency:(a) vary any conditions of the certificate;(b) omit any conditions of the certificate;(c) impose new conditions in the certificate.
12. Cancellation or suspension of certificate of competency
(1) The Registrar may, by notice in writing to the holder of a certificate of competency, cancel or suspend the certificate if –(a) the holder has not complied with a condition of the certificate; or(b) the holder has been convicted of any offence under any Act if the Registrar considers the offence to be relevant to the holder's competency to handle a listed chemical product; or(c) the annual fee payable under regulation 15 has not been paid on or before the date on which it is due; or(d) the Registrar becomes aware of any information which, if it had been known at the time when the certificate was granted, would have prevented the grant of the certificate; or(e) the Registrar is satisfied that the holder is unable to competently and safely handle or use a listed chemical product, or class of listed chemical products, to which the certificate relates.(2) A notice issued under subregulation (1) must specify –(a) the date on which the cancellation or suspension takes effect; and(b) in the case of suspension –(i) the period of suspension; or(ii) any conditions that must be satisfied before the suspension is lifted.
13. Notice of reasons and opportunity to comment
Before suspending or cancelling a certificate of competency or refusing to grant an application for a certificate of competency, the Registrar must give the holder of, or the applicant for, the certificate –(a) notice of the reasons for the proposed action; and(b) the opportunity to submit written comments on the proposed action.
14. Term of certificate of competency
A certificate of competency continues in operation for the term (not exceeding 5 years) specified in the certificate, unless it is earlier cancelled.
15. Payment of annual fee for certificate of competency
The holder of a certificate of competency must pay the prescribed fee each year on or before the yearly anniversary of the granting of the certificate.
16. Refusal to grant authority
For the purposes of clause 3(4)(b) of Part 1 of Schedule 5 to the Act, the following grounds are prescribed as grounds on which the Registrar may refuse to grant an authority, within the meaning of that Schedule:(a) the fact that the applicant for such an authority is not the holder of a certificate of competency in relation to the listed chemical product proposed to be handled under that authority;(b) the fact that the applicant is under 18 years of age.
PART 3 - Special Precautions for Handling and Storing Schedule 6 or 7 Poison
In this Part –concentrate means a Schedule 6 or 7 poison that for the purpose of its use is intended to be diluted or mixed with water or another diluent;enclosed building means a building, erection or tunnel in which the free movement of air is controlled or impeded in any manner;protective clothing and equipment means the clothing and equipment required by this Part to be worn or used by a chemical operator;respirator means an apparatus that covers the mouth and nose of a person and is designed to –(a) ensure the person of a supply of air adequate for respiration; and(b) eliminate as far as practicable the risk of pollution, by a Schedule 6 or 7 poison, of the air that is breathed by the person;Schedule 6 or 7 poison means a chemical product that is listed, or which contains a constituent that is listed, in Schedule 6 or 7 to the current Poisons Standard;spray operation means agricultural spraying involving the use of a Schedule 6 or 7 poison.
18. Storage of Schedule 6 or 7 poison
A person who has possession or control of a Schedule 6 or 7 poison must ensure that it is not readily accessible, when being kept, to any person whom he or she has not authorised to have access to it.Penalty: Fine not exceeding 50 penalty units.
19. Condition of clothing and equipment generally
A person must not wear or use protective clothing and equipment unless –(a) it is in good order and condition; and(b) it is ready for use for the purpose for which it is intended.Penalty: Fine not exceeding 20 penalty units.
20. General duties of employer
A person who employs another to handle a Schedule 6 or 7 poison must –(a) ensure that the employee is provided with the protective clothing, facilities, equipment and materials necessary to enable the Schedule 6 or 7 poison to be handled in accordance with this Part; and(b) make a copy of this Part readily available to the employee; and(c) take all reasonable steps to ensure that the employee is aware of the provisions of this Part relevant to the handling of the Schedule 6 or 7 poison; and(d) take all reasonable steps to ensure that the employee complies with this Part when handling the Schedule 6 or 7 poison.Penalty: Fine not exceeding 50 penalty units.
21. General duty of chemical operator handling Schedule 6 or 7 poison
If this Part requires that any clothing, facility, material or equipment be provided to, or for the use of, a chemical operator in respect of the handling of a Schedule 6 or 7 poison, the chemical operator must not handle the Schedule 6 or 7 poison unless that clothing, facility, material or equipment has been provided.Penalty: Fine not exceeding 20 penalty units.
(1) At all times while handling a Schedule 6 or 7 poison a chemical operator must wear –(a) clean cotton overalls; and(b) a hat; and(c) boots.Penalty: Fine not exceeding 20 penalty units.(2) If the chemical operator is diluting or mixing a concentrate or transferring a concentrate from one container to another, the chemical operator must also wear protective gloves and a face shield.Penalty: Fine not exceeding 20 penalty units.(3) If the chemical operator is handling a Schedule 6 or 7 poison that is in the form of a fine dust that is liable to be blown about, the chemical operator must also wear protective gloves and a respirator.Penalty: Fine not exceeding 20 penalty units.(4) If the chemical operator is engaged in spray operations, the chemical operator must also wear protective gloves and a respirator.Penalty: Fine not exceeding 20 penalty units.(5) If the chemical operator is handling a Schedule 6 or 7 poison in an enclosed building, the chemical operator must also wear a respirator.Penalty: Fine not exceeding 20 penalty units.(6) After handling a Schedule 6 or 7 poison, the chemical operator must ensure that any protective clothing which has come into contact with the Schedule 6 or 7 poison during that handling is cleaned of that poison before the protective clothing is next put on by any person.Penalty: Fine not exceeding 20 penalty units.
(1) A plentiful supply of soap and clean water must be provided for the use of a chemical operator in a place where the chemical operator dilutes or mixes a concentrate or transfers a concentrate from one container to another.(2) A plentiful supply of clean water must be provided for the use of a chemical operator in a place where the chemical operator performs spray operations.
24. Dealing with Schedule 6 or 7 poison on skin or clothing
(1) If a Schedule 6 or 7 poison is splashed or spilt on the skin of a chemical operator, the chemical operator is to immediately wash thoroughly with clean water the area of skin affected.(2) If a Schedule 6 or 7 poison is splashed or spilt on the clothing of a chemical operator to the extent that the clothing may fail to prevent the skin of the chemical operator from being affected, the chemical operator is to immediately remove that clothing and wash thoroughly with clean water any skin that may have been affected by the splash or spill.
PART 4 - Hormonal Growth Promotants
25. Obtaining and keeping copy of hormonal growth promotant purchaser declaration
If a person signs a hormonal growth promotant purchaser declaration –(a) the supplier of the hormonal growth promotant must provide that person with a copy of the declaration; and(b) that person must keep the copy for at least 2 years.Penalty: Fine not exceeding 50 penalty units.
26. Possession of hormonal growth promotant
A person must not have possession or custody of a hormonal growth promotant unless the person –(a) has possession or custody of the hormonal growth promotant as a supplier and has a unique notification number within the meaning of regulation 47(1) of the Agvet Regulations of Tasmania; or(b) is employed by the holder of such a unique notification number and has possession or custody of the hormonal growth promotant in the course of that employment; or(c) has signed a hormonal growth promotant purchaser declaration; or(d) is employed by a person who has signed such a declaration and has possession or custody of the hormonal growth promotant in the course of that employment; or(e) has possession or custody of the hormonal growth promotant while under the supervision of a person who has signed such a declaration.Penalty: Fine not exceeding 100 penalty units.
27. Treatment of animals with hormonal growth promotant
(1) In this regulation –property identification code, in respect of a hormonal growth promotant, means the property identification code, within the meaning of the Animal (Brands and Movement) Regulations 2014 , relating to the property specified in the hormonal growth promotant purchaser declaration.(2) A person must not implant a hormonal growth promotant into an animal that is not capable of bearing a tail tag, with the property reference number for the hormonal growth promotant, if the person intends the animal to be sold at a saleyard, or sent to an abattoir, immediately after the implant.Penalty: Fine not exceeding 100 penalty units.
28. Earmark on hormonal growth promotant-treated animal
(1) In this regulation –registered earmark means an earmark which has been registered under the Animal (Brands and Movement) Act 1984 in respect of the identification of an animal implanted with a hormonal growth promotant.(2) If a person implants a hormonal growth promotant into an animal that has not previously been implanted with a hormonal growth promotant, the person must apply a registered earmark to the ear of the animal at the time of implanting the hormonal growth promotant.Penalty: Fine not exceeding 100 penalty units.
29. Record of hormonal growth promotant use
A person who implants a hormonal growth promotant in an animal must –(a) keep a record of the purchase and use of the hormonal growth promotant in a form approved by the Registrar; and(b) keep the record for at least 2 years.Penalty: Fine not exceeding 50 penalty units.
30. Duty of person who intends to obtain HGP-Free tag
A person must not obtain an HGP-Free tag for attachment to an animal unless he or she has first –(a) completed, signed and provided, to the Registrar of Animal Brands, an HGP-Free Declaration; and(b) completed, and provided to the supplier of the tag, an authorised order.Penalty: Fine not exceeding 100 penalty units.
31. Registrar of Animal Brands to provide authorised order form
(1) On receipt of an HGP-Free Declaration under regulation 30(a) , the Registrar of Animal Brands must, if the declaration appears to that Registrar to be validly made, provide to the person who made the declaration an authorised order form which contains –(a) the property identification code, that appears on a brand or tag registered under the Animal (Brands and Movement) Act 1984 , of the property which that person, or another person of whom that person is the authorised representative, is the proprietor; and(b) the name of that proprietor; and(c) an endorsement by the Registrar of Animal Brands or his or her representative to the effect that the declaration has been provided to that Registrar.(2) An authorised order form provided under subregulation (1) is to be in a form approved by the Registrar of Animal Brands.
32. Suppliers of HGP-Free tags
The Secretary may approve, by notice published in the Gazette, persons, or classes of persons, who may supply HGP-Free tags.
(1) A person must not supply an HGP-Free tag to another person unless that supplier is a person approved under regulation 32 or a person of a class of persons approved under that regulation.Penalty: Fine not exceeding 100 penalty units.(2) A supplier of HGP-Free tags must not supply an HGP-Free tag to a person unless that person has provided to that supplier a completed authorised order form.Penalty: Fine not exceeding 100 penalty units.(3) It is a defence to a charge for an offence under subregulation (2) for the supplier to show that the supplier had received a completed form which the supplier had reason to believe was an authorised order form.
34. Application of HGP-Free tag
(1) A person may only attach, or assist in attaching, an HGP-Free tag to an animal if –(a) the person applying that tag has signed, or is acting under the supervision or direction of a person who has signed, an HGP-Free Declaration which relates to an identification number for the property that would appear on a tail tag on the animal if the animal were to be sold at a saleyard, or sent to an abattoir, immediately after the HGP-Free tag were attached; and(b) the period, if any, declared by the Secretary by notice in a newspaper circulating in the State has not elapsed since that declaration was signed; and(c) the person who has signed that HGP-Free Declaration –(i) has bred the animal, or acquired the animal before it was 6 weeks old, and has not implanted a hormonal growth promotant in the animal; or(ii) has not implanted a hormonal growth promotant in the animal since assuming responsibility for the husbandry of the animal and has documentary evidence that the animal was not implanted with a hormonal growth promotant before that person assumed that responsibility; and(d) the person does not have reason to believe that the animal has been implanted with a hormonal growth promotant.Penalty: Fine not exceeding 100 penalty units.(2) For the purposes of subregulation (1)(c)(ii) , the following documents are documentary evidence:(a) a purchaser invoice provided by a stock agent under regulation 38(1) ;(b) a declaration made under regulation 35(1) by the owner from whom the animals were obtained or other person responsible for the husbandry of the animals on behalf of that owner.
35. HGP-Free Declaration at time of disposal
(1) The owner of animals who intends to sell or otherwise dispose of them, or a person who has been responsible for the husbandry of the animals, may only declare that the animals have not been implanted with a hormonal growth promotant at any time if that owner or person –(a) bred the animals, or acquired the animals before they were 6 weeks old, and has not implanted a hormonal growth promotant in the animals; or(b) has not implanted a hormonal growth promotant in the animal since assuming responsibility for the husbandry of the animal and has documentary evidence that the animal was not implanted with a hormonal growth promotant before that person assumed that responsibility.Penalty: Fine not exceeding 100 penalty units.(2) For the purposes of subregulation (1)(b) , the following documents are documentary evidence:(a) a purchaser invoice provided by a stock agent under regulation 38(1) ;(b) a declaration made under subregulation (1) by the owner from whom the animals were obtained or other person responsible for the husbandry of the animals on behalf of that owner.(3) A declaration under subregulation (1) is to be in accordance with –(a) a national vendor declaration; or(b) a form approved by the Registrar.
36. Records to be kept in respect of hormonal growth promotant-free animal
A person who has –must keep a record of the obtaining and sale, or other disposal, of that animal for a period of at least 2 years after the animal has been sold or disposed of.(a) made an HGP-Free Declaration; or(b) obtained an animal with documentary evidence, within the meaning of regulation 35(2) , that the animal has not been implanted with a hormonal growth promotant –Penalty: Fine not exceeding 20 penalty units.
37. Power of inspector to seize HGP-Free tag
An inspector may seize and retain an HGP-Free tag if the inspector believes on reasonable grounds that it is in the possession of a person who –(a) has not signed an HGP-Free Declaration; or(b) is not employed by, or under the supervision or direction of, a person referred to in paragraph (a); or(c) has improperly disposed of other HGP-Free tags; or(d) has attached other HGP-Free tags in contravention of the Act or these regulations.
38. Responsibilities of stock agent
(1) A stock agent who sells an animal that bears an HGP-Free tag must provide the purchaser with a purchaser invoice containing the following information and statements:(a) the date of sale of the animal;(b) the name of the stock agent who sold the animal;(c) the place where the animal is sold;(d) the number on the HGP-Free tag on the animal;(e) a description of the animal;(f) a statement to the effect that the animal listed on the invoice was tagged with an HGP-Free tag and, if the statement is in writing, signed by the stock agent.Penalty: Fine not exceeding 50 penalty units.(2) A stock agent must, if he or she has had possession or custody of an animal that has been implanted with a hormonal growth promotant or has been tagged with an HGP-Free tag –(a) allow an inspector at any reasonable time to inspect and make copies of, or take extracts from, the records relating to that animal; and(b) answer any questions asked by, and provide any information requested by, an inspector in relation to that animal and those records; and(c) provide an inspector with reasonable assistance.Penalty: Fine not exceeding 50 penalty units.
PART 5 - Miscellaneous
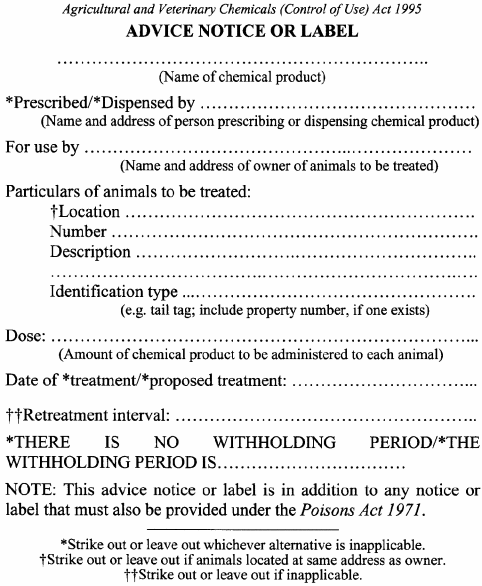
For the purposes of section 18(1)(d) of the Act, an advice notice or label is to be in accordance with Form 1.
40. Provision of advice notice or label
When supplying a veterinary chemical product to a person, the veterinary surgeon or the person who supplies the veterinary chemical product in compliance with the prescription of a veterinary surgeon is also to provide that person with the advice notice or label required under section 18(1)(d) of the Act relating to the veterinary chemical product.
41. Records to be kept by veterinary surgeon
If a veterinary surgeon uses or supplies, or supplies a prescription for, a veterinary chemical product prepared by a veterinary surgeon or registered pharmaceutical chemist, the veterinary surgeon is to make a record containing the following information and keep that record for at least 2 years:(a) the name and address of the owner of the animal treated or to be treated;(b) a description of the animal;(c) the date on which the animal was treated if the veterinary surgeon treats the animal with the veterinary chemical product;(d) the directions for use of the veterinary chemical product if the veterinary surgeon supplies, or writes a prescription for, the veterinary chemical product;(e) the name, strength and form of the veterinary chemical product;(f) the withholding period, if any, as specified in the advice notice or label required under section 18(1)(d) of the Act that accompanied the veterinary chemical product.
42. Prescribed information, &c., for purposes of section 37 of Act
(1) In this regulation –Schedule 5 poison means an agricultural chemical product that is listed, or that contains a constituent listed, in Schedule 5 to the current Poisons Standard;Schedule 6 poison means an agricultural chemical product that is listed, or that contains a constituent listed, in Schedule 6 to the current Poisons Standard;Schedule 7 poison means an agricultural chemical product that is listed, or that contains a constituent listed, in Schedule 7 to the current Poisons Standard;(2) For the purposes of sections 37(1)(a) , (b) and (c) of the Act –(a) any Schedule 5 poison, Schedule 6 poison or Schedule 7 poison is a prescribed agricultural chemical product; and(b) in the case of a Schedule 5 poison, the prescribed information is –(i) the name of the agricultural chemical product that is the Schedule 5 poison; and(ii) a copy of the label approved under the Code in relation to that agricultural chemical product; and(c) in the case of a Schedule 6 poison or Schedule 7 poison, the prescribed information is –(i) the name of the agricultural chemical product that is the Schedule 6 poison or Schedule 7 poison; and(ii) a copy of the label approved under the Code in relation to that agricultural chemical product; and(iii) a copy of any material safety data sheet that relates to that agricultural chemical product; and(d) the prescribed manner is the manner specified in the directions for use in the label approved under the Code in relation to the agricultural chemical product that is the Schedule 5 poison, Schedule 6 poison or Schedule 7 poison.
(1) It is a condition of the supply or use of any agricultural produce intended for use as stockfood that the level of any substance in the agricultural produce must not exceed the maximum permitted concentration.(2) It is a condition of the supply or use of any agricultural produce intended for use as stockfood that the level of any residue of a chemical product in the agricultural produce must not exceed –(a) the level for the chemical product shown against the appropriate feed commodity in the Animal Feedstuffs Residue Standard; or(b) if no such level is shown in that Standard, the level which the Registrar has determined by –(i) notice published in the Gazette or a newspaper circulating in the State; or(ii) written notice provided to the supplier or user.(3) A person who contravenes a condition specified in this regulation is guilty of an offence and liable on summary conviction to –(a) in the case of a condition specified under subregulation (1) , a penalty of a fine not exceeding 100 penalty units; or(b) in the case of a condition specified under subregulation (2) , a penalty of a fine not exceeding 50 penalty units.
44. Prescribed level of residue which adversely affects plants, stock &c.
(1) In this regulation –Drinking Water Guidelines means the Australian Drinking Water Guidelines 6 (2011), published by the National Health and Medical Research Council established by the National Health and Medical Research Council Act 1992 of the Commonwealth, as amended or substituted from time to time.(2) The following levels of residue are the prescribed levels for the purposes of the definition of adversely affects in section 30(3) of the Act:(a) in the case of stock and agricultural produce, the maximum residue limit;(b) in the case of water used as or to provide drinking water for stock, other animals and humans, the level relating to the stock, other animals or humans specified in the Drinking Water Guidelines;(c) in the case of groundwater or soil, the level which the Registrar determines in relation to the whole State, a part of the State or an area will likely result in the contamination of agricultural produce that is derived from one or more of the following:(i) plants grown in the soil in the State, that part of the State or that area;(ii) stock in contact with the soil in the State, that part of the State or that area;(iii) stock or plants in contact with the groundwater in the State, that part of the State or that area;(d) in the case of premises, the level which the Registrar determines in relation to the whole State, part of the State or an area will likely result in –(i) the contamination of agricultural produce that is stored in, processed in or otherwise in contact with the premises; or(ii) rendering anything on those premises unsuitable for its normal use;(e) in the case of agricultural produce intended for use as stockfood, the level which the Registrar determines in relation to the whole State, a part of the State or an area is likely to result in the contamination of stock fed on that stockfood.(3) If the Registrar makes a determination under subregulation (2)(c) , (d) or (e) , notification of the determination is to be published in the Gazette or in a newspaper circulating in the whole State, the part of the State or the area in relation to which the determination is made.
45. Testing at expense of owner
For the purposes of section 49(2)(e)(i) of the Act, the following reasons are prescribed as reasons for requiring testing of samples or specimens of any stock, premises, agricultural produce, preparation or fitting to be carried out at the expense of the owner:(a) that, at any time during the period of one year ending on the giving of the notice requiring the testing, one or more of the following has occurred:(i) the owner has supplied, or consigned for slaughter, contaminated stock;(ii) the owner has supplied any contaminated agricultural produce, preparation or fitting;(iii) the owner has been found guilty of an offence under section 18 , 20 , 25 , 28 , 30 , 31 , 32 , 40 , 41 , 42 , 43 or 46 of the Act or any regulation made in respect of any of those sections and relating to the handling of a chemical product;(iv) the owner has been found guilty of an offence under the Poisons Act 1971 in relation to the use or possession of a chemical product that is listed, or which contains a constituent that is listed, in Schedule 4 to the current Poisons Standard and was prescribed by a veterinary surgeon for use in relation to an animal;(b) that the owner owns or occupies premises subject to a premises use restriction notice and the testing is reasonably required to determine whether –(i) the owner or other occupier of the premises is meeting the requirements of that notice; or(ii) that notice should remain in force.
46. False or misleading statements about residues
A person must not make, or cause to be made, a statement relating to the presence or likelihood of the presence of a residue, or a substance for which a maximum permitted concentration is declared, in any animal or agricultural produce if the person knows or believes the statement or representation is false or misleading in a material particular.Penalty: Fine not exceeding 100 penalty units.
SCHEDULE 1 - Listed Chemical Products
1. Any agricultural chemical product containing methyl bromide.
2. Any agricultural chemical product when used in the delivery of a service offered for fee or other reward.
3. Any veterinary chemical product when used in the delivery of a service offered for fee or other reward.
4. Any agricultural chemical product containing phosphine.
SCHEDULE 2 - Form
Form 1
SCHEDULE 3 - Fees
PART 1 - Authorities
Fee units | 1. | Application for permit to handle a chemical product which is not registered under the Code | 50 | 2. | Application for permit to handle a chemical product, which is registered under the Code, otherwise than in accordance with the label approved under the Code | 50 | 3. | Application for permit to handle a chemical product where the handling of that chemical product is prohibited under section 20(2)(b) of the Act | 50 | 4. | Annual fee for a permit referred to in item 3 | 50 | 5. | Application for commercial chemical operator licence | 100 | 6. | Annual fee for commercial chemical operator licence | 100 | 7. | Application for restricted chemical product permit | 20 | 8. | Annual fee for restricted chemical product permit | 20 | 9. | Application for agricultural spraying permit – | (a) if the permit is required to permit spraying otherwise prohibited by clause 5 of the Agricultural and Veterinary Chemicals (Control of Use) (Agricultural Spraying) Order 1996 | 60 | (b) if the permit is required to permit spraying otherwise prohibited by clause 6 of the Agricultural and Veterinary Chemicals (Control of Use) (Agricultural Spraying) Order 1996 | 0 | (c) if the permit is required to permit spraying otherwise prohibited by clause 7 of the Agricultural and Veterinary Chemicals (Control of Use) (Agricultural Spraying) Order 1996 | 60 | 10. | Application for pilot (chemical rating) licence where the applicant holds a current agricultural aircraft operator licence | 20 | 11. | Annual fee for a licence referred to in item 10 | 20 | 12. | Application for pilot (chemical rating) licence where the applicant does not hold a current agricultural aircraft operator licence | 100 | 13. | Annual fee for a licence referred to in item 12 | 100 | 14. | Agricultural aircraft operator licence | 100 | 15. | Annual fee for agricultural aircraft operator licence | 100 |
PART 2 - Certificates of Competency
Fee units | 1. | Application for category 1 chemical user certificate of competency | 10 | 2. | Annual fee for category 1 chemical user certificate of competency | 10 | 3. | Application for category 2 chemical user certificate of competency | 40 | 4. | Annual fee for category 2 chemical user certificate of competency | 40 | 5. | Application for category 3 chemical user certificate of competency | 40 | 6. | Annual fee for category 3 chemical user certificate of competency | 40 | 7. | Application for pest management technician certificate of competency | 50 | 8. | Annual fee for pest management technician certificate of competency | 50 | 9. | Application for methyl bromide or phosphine user certificate of competency | 20 | 10. | Annual fee for methyl bromide or phosphine user certificate of competency | 20 |
PART 3 - Other Fees
Fee units | 1. | Application for direction under section 31(1) or (2) of the Act | 50 | 2. | Supply of information about premises use restriction notices under section 44(1) of the Act | 50 |
Displayed and numbered in accordance with the Rules Publication Act 1953.
Notified in the Gazette on 20 April 2022
These regulations are administered in the Department of Natural Resources and Environment Tasmania.